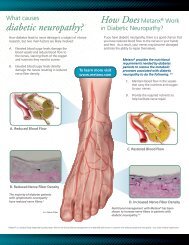
Download - ADVANCE for NPs & PAs
Download - ADVANCE for NPs & PAs
Download - ADVANCE for NPs & PAs
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
(fentanyl buccal tablet, Cephalon, Inc., Frazer, Pennsylvania) andACTIQ® (OTFC lozenge, Cephalon, Inc., Frazer, Pennsylvania). 6REMS programs meeting the new FDA requirements have beenapproved <strong>for</strong> the fentanyl sublingual tablet (Abstral®, ProStrakan,Inc., Bedminster, New Jersey) and fentanyl nasal spray (Lazanda®,Archimedes Development Ltd, Nottingham, UK). Revisions to theexisting REMS <strong>for</strong> fentanyl buccal soluble lm (Onsolis®, MedaPharmaceuticals, Inc., Somerset, New Jersey) are under review asof this writing. 3 To date, FDA has not required REMS programs <strong>for</strong>non-TIRF, short-acting opioid products.Breakthrough Pain in Cancer and NoncancerPatients: An OverviewBY ARVIND NARAYANA, MDCEPHALON, INC. | FRAZER, PENNSYLVANIAPatients with chronic pain often experience transitory exacerbationsof pain despite long-term treatment with anaround-the-clock opioid. These exacerbations, or ares, thatoccur on a background of otherwise well-controlled, persistent,chronic pain are dened as BTP. 7 The prevalence of BTP is high; in arecent survey of community-dwelling patients with chronic canceror noncancer pain, 33% and 48%, respectively, experienced BTP. 8In patients with chronic pain who have controlled persistent pain,the presence of BTP has been associated with increased healthcarecosts due to hospitalizations and emergency department visits, 9and patients with BTP have greater functional impairment, disability,depression, and anxiety, as well as poorer quality of life thanpatients without BTP. 10,11Many BTP episodes reach peak intensity within minutes, farsooner than the onset of analgesia produced with conventional,orally administered, short-acting opioid medications (30 to 60minutes). 11-13 TIRF products have been developed to address theneed <strong>for</strong> opioids with a rapid onset of analgesia that more closelymatches the time prole of a typical BTP episode.FENTANYL BUCCAL TABLET: CLINICAL DATA REVIEWThe fentanyl buccal tablet is a rapid-onset, schedule II opioid approvedby the U.S. Food and Drug Administration in 2006 <strong>for</strong> thetreatment of BTP in patients with cancer who are already receivingand are tolerant to opioid therapy <strong>for</strong> their underlying persistentcancer pain. 14 Fentanyl buccal tablet utilizes OraVescent® drugdelivery technology (CIMA Labs, Inc., Eden Prairie, Minnesota),which employs a chemical reaction to enhance the rate and extentof absorption of fentanyl through the buccal mucosa. 15The clinical efficacy and safety program <strong>for</strong> fentanyl buccal tabletencompasses 8 well-designed, peer-reviewed, published clinicalstudies. Three of these studies included patients with cancerrelatedBTP, 16-18 and 5 included patients with noncancer-related BTP(e.g., neuropathic or low back pain). 19-23 The analgesic response toFBT in these studies (as measured by the percentage of episodesof BTP with a ≥33% improvement in pain intensity) is presented inFigure 1. In general, the inclusion and exclusion criteria in thesestudies are representative of the patient selection standards thatcan be employed in clinical practice. Each study included opioidtolerantpatients who were receiving around-the-clock opioids <strong>for</strong>their controlled persistent pain. 16-23 In some studies, patients hadto have an average pain intensity score of ≤6 (or in some studies,