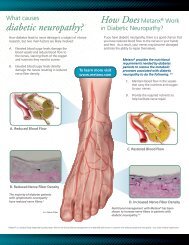
Download - ADVANCE for NPs & PAs
Download - ADVANCE for NPs & PAs
Download - ADVANCE for NPs & PAs
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Application site reactions or abnormalities were reported in 12%of patients; these incidences tended to occur early in treatment,were transitory, and led to study discontinuation in approximately1% of patients. Serious adverse events that were considered possiblyrelated or directly related to study drug were reported in 2of the 3 double-blind, placebo-controlled studies described earlier.One patient experienced accidental overdose resulting in a loss ofconsciousness. 20 This patient was admitted to the hospital and fullyrecovered. Another patient experienced pneumonia and had anaccidental overdose of opioid medication, and in another patient,abuse of opioid medications resulting in withdrawal symptomswas reported. 21 During the long-term safety study, serious adverseevents occurred in 18% of patients, the most common of whichincluded chest pain, pneumonia, and vomiting (5 patients each). 23ORAL TRANSMUCOSAL FENTANYL CITRATE:CLINICAL DATA REVIEWOTFC is a TIRF <strong>for</strong>mulation approved by the Food and DrugAdministration in 1998 <strong>for</strong> the treatment of BTP in patients withcancer who are already receiving and are tolerant to around-theclockopioid therapy <strong>for</strong> their underlying persistent cancer pain. 27Each OTFC lozenge consists of fentanyl, incorporated in a sweetenedmatrix and attached to a handle. The patient administersOTFC by rubbing the lozenge along the inside of the cheek, whichcauses the lozenge to dissolve in the mouth, allowing rapid absorptionof fentanyl through the buccal mucosa. 28The efficacy and safety of OTFC was assessed in a randomized,double-blind, placebo-controlled study of opioid-tolerant patientswith cancer. 29 After an open-label titration period to identify aneffective dose of OTFC, 92 patients entered a double-blind, crossoverphase, where they were given 10 randomly ordered treatments(7 doses of OTFC at the effective dose identied and 3doses of placebo) in the <strong>for</strong>m of identical lozenges to treat 10 BTPepisodes. Signicant differences in pain intensity were observedat all time points after OTFC administration compared with placebo(P