Staff Members of the Institute of Biochemistry, TU - Institut für ...
Staff Members of the Institute of Biochemistry, TU - Institut für ...
Staff Members of the Institute of Biochemistry, TU - Institut für ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
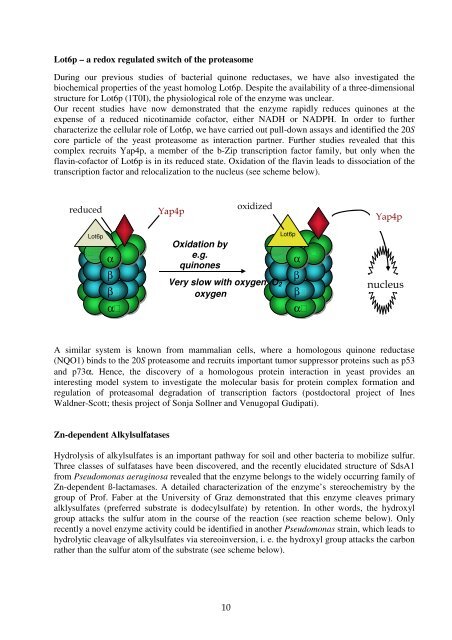
Lot6p – a redox regulated switch <strong>of</strong> <strong>the</strong> proteasome<br />
During our previous studies <strong>of</strong> bacterial quinone reductases, we have also investigated <strong>the</strong><br />
biochemical properties <strong>of</strong> <strong>the</strong> yeast homolog Lot6p. Despite <strong>the</strong> availability <strong>of</strong> a three-dimensional<br />
structure for Lot6p (1T0I), <strong>the</strong> physiological role <strong>of</strong> <strong>the</strong> enzyme was unclear.<br />
Our recent studies have now demonstrated that <strong>the</strong> enzyme rapidly reduces quinones at <strong>the</strong><br />
expense <strong>of</strong> a reduced nicotinamide c<strong>of</strong>actor, ei<strong>the</strong>r NADH or NADPH. In order to fur<strong>the</strong>r<br />
characterize <strong>the</strong> cellular role <strong>of</strong> Lot6p, we have carried out pull-down assays and identified <strong>the</strong> 20S<br />
core particle <strong>of</strong> <strong>the</strong> yeast proteasome as interaction partner. Fur<strong>the</strong>r studies revealed that this<br />
complex recruits Yap4p, a member <strong>of</strong> <strong>the</strong> b-Zip transcription factor family, but only when <strong>the</strong><br />
flavin-c<strong>of</strong>actor <strong>of</strong> Lot6p is in its reduced state. Oxidation <strong>of</strong> <strong>the</strong> flavin leads to dissociation <strong>of</strong> <strong>the</strong><br />
transcription factor and relocalization to <strong>the</strong> nucleus (see scheme below).<br />
reduced<br />
Lot6p<br />
α<br />
β<br />
β<br />
α<br />
A similar system is known from mammalian cells, where a homologous quinone reductase<br />
(NQO1) binds to <strong>the</strong> 20S proteasome and recruits important tumor suppressor proteins such as p53<br />
and p73α. Hence, <strong>the</strong> discovery <strong>of</strong> a homologous protein interaction in yeast provides an<br />
interesting model system to investigate <strong>the</strong> molecular basis for protein complex formation and<br />
regulation <strong>of</strong> proteasomal degradation <strong>of</strong> transcription factors (postdoctoral project <strong>of</strong> Ines<br />
Waldner-Scott; <strong>the</strong>sis project <strong>of</strong> Sonja Sollner and Venugopal Gudipati).<br />
Zn-dependent Alkylsulfatases<br />
Yap4p<br />
Oxidation by<br />
e.g.<br />
quinones<br />
Hydrolysis <strong>of</strong> alkylsulfates is an important pathway for soil and o<strong>the</strong>r bacteria to mobilize sulfur.<br />
Three classes <strong>of</strong> sulfatases have been discovered, and <strong>the</strong> recently elucidated structure <strong>of</strong> SdsA1<br />
from Pseudomonas aeruginosa revealed that <strong>the</strong> enzyme belongs to <strong>the</strong> widely occurring family <strong>of</strong><br />
Zn-dependent ß-lactamases. A detailed characterization <strong>of</strong> <strong>the</strong> enzyme’s stereochemistry by <strong>the</strong><br />
group <strong>of</strong> Pr<strong>of</strong>. Faber at <strong>the</strong> University <strong>of</strong> Graz demonstrated that this enzyme cleaves primary<br />
alklysulfates (preferred substrate is dodecylsulfate) by retention. In o<strong>the</strong>r words, <strong>the</strong> hydroxyl<br />
group attacks <strong>the</strong> sulfur atom in <strong>the</strong> course <strong>of</strong> <strong>the</strong> reaction (see reaction scheme below). Only<br />
recently a novel enzyme activity could be identified in ano<strong>the</strong>r Pseudomonas strain, which leads to<br />
hydrolytic cleavage <strong>of</strong> alkylsulfates via stereoinversion, i. e. <strong>the</strong> hydroxyl group attacks <strong>the</strong> carbon<br />
ra<strong>the</strong>r than <strong>the</strong> sulfur atom <strong>of</strong> <strong>the</strong> substrate (see scheme below).<br />
10<br />
oxidized<br />
Lot6p<br />
Very slow with oxygen O2 nucleus<br />
oxygen<br />
β<br />
α<br />
β<br />
α<br />
Yap4p<br />
Yap4p