Staff Members of the Institute of Biochemistry, TU - Institut für ...
Staff Members of the Institute of Biochemistry, TU - Institut für ...
Staff Members of the Institute of Biochemistry, TU - Institut für ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Staff</strong> <strong>Members</strong> <strong>of</strong> <strong>the</strong> <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong>, <strong>TU</strong> Graz<br />
http://www.biochemistry.tugraz.at/<br />
Pr<strong>of</strong>essors<br />
Peter Macheroux (Full Pr<strong>of</strong>essor & Head <strong>of</strong> <strong>the</strong> <strong><strong>Institut</strong>e</strong>)<br />
(peter.macheroux@tugraz.at; Tel.: +43-(0)316-873-6450)<br />
Gün<strong>the</strong>r Daum (Associate Pr<strong>of</strong>essor)<br />
(guen<strong>the</strong>r.daum@tugraz.at; Tel.: +43-(0)316-873-6462)<br />
Albin Hermetter (Associate Pr<strong>of</strong>essor)<br />
(albin.hermetter@tugraz.at; Tel.: +43-(0)316-873-6457)<br />
Michael Murkovic (Associate Pr<strong>of</strong>essor)<br />
michael.murkovic@tugraz.at; Tel.: +43-(0)316-873-6495)<br />
Karin A<strong>the</strong>nstaedt (Independent Group Leader)<br />
(karin.a<strong>the</strong>nstaedt@tugraz.at; Tel.:+43-(0)316-873-6460)<br />
Assistants<br />
Dr. Ines Waldner-Scott<br />
(ines.waldner-scott@tugraz.at; Tel.:+43-(0)316-873-6454)<br />
DI Tanja Knaus<br />
(tanja.knaus@tugraz.at; Tel.:+43-(0)316-873-6463)<br />
DI Alexandra Binter<br />
(alexandra.binter@tugraz.at; Tel.: +43-(0)316-873-6453)<br />
DI Silvia Wallner<br />
(silvia.wallner@tugraz.at; Tel.: +43-(0)316-873-6955)<br />
Office<br />
Annemarie Portschy<br />
(portschy@tugraz.at; Tel.: +43-(0)316-873-6451; Fax: +43-(0)316-873-6952)<br />
Technical <strong>Staff</strong><br />
Claudia Hrastnik; claudia.hrastnik@tugraz.at; Tel.: +43-(0)316-873-6460<br />
Steve Stipsits; steve.stipsits@tugraz.at; Tel.: 43-(0)316-873 6464<br />
Rosemarie Trenker-El-Toukhy; r.trenker-el-toukhy@tugraz.at; Tel.: +43-(0)316-873-6464<br />
Elfriede Zenzmaier; elfriede.zenzmaier@tugraz.at; Tel.: +43-(0)316-873-6467<br />
Leo H<strong>of</strong>er (Workshop); leo.h<strong>of</strong>er@tugraz.at; Tel.: +43-(0)316-873-8431 or 8433<br />
1
Brief History <strong>of</strong> <strong>the</strong> <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong><br />
The <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong> and Food Chemistry was born out <strong>of</strong> <strong>the</strong> division <strong>of</strong> <strong>the</strong> <strong><strong>Institut</strong>e</strong><br />
<strong>of</strong> Biochemical Technology, Food Chemistry and Microchemistry <strong>of</strong> <strong>the</strong> former School <strong>of</strong><br />
Technology Graz. Toge<strong>the</strong>r with all <strong>the</strong> o<strong>the</strong>r chemistry institutes, it was located in <strong>the</strong> old<br />
Chemistry Building on Baron Mandell's ground, corner Technikerstrasse-Mandellstrasse.<br />
1929 The <strong><strong>Institut</strong>e</strong> <strong>of</strong> Technical <strong>Biochemistry</strong> and Microbiology moved to <strong>the</strong> building <strong>of</strong><br />
<strong>the</strong> Fürstlich-Dietrichstein-Stiftung, Schlögelgasse 9, in which all <strong>the</strong> biosciences were<br />
<strong>the</strong>n concentrated.<br />
1945 Georg GORBACH - initially in <strong>the</strong> rank <strong>of</strong> a docent and soon <strong>the</strong>reafter as a.o.<br />
Pr<strong>of</strong>essor - took over to lead <strong>the</strong> institute. The institute was renamed <strong><strong>Institut</strong>e</strong> <strong>of</strong><br />
Biochemical Technology and Food Chemistry.<br />
1948 G. GORBACH was nominated full pr<strong>of</strong>essor and head <strong>of</strong> <strong>the</strong> institute. In succession <strong>of</strong><br />
<strong>the</strong> famous Graz School <strong>of</strong> Microchemistry founded by PREGL and EMICH, Pr<strong>of</strong>.<br />
GORBACH was one <strong>of</strong> <strong>the</strong> most prominent and active leaders in <strong>the</strong> fields <strong>of</strong><br />
microchemistry, microbiology and nutritional sciences. After World War II, questions<br />
<strong>of</strong> water quality and waste water disposal became urgent; hence, <strong>the</strong> group <strong>of</strong> Pr<strong>of</strong>. K.<br />
S<strong>TU</strong>NDL, which at that time was part <strong>of</strong> <strong>the</strong> institute, was gaining importance. In<br />
addition, a division to fight dry-rot supervised by Dr. KUNZE and after his demise by<br />
H. SALOMON, was also affiliated with <strong>the</strong> institute.<br />
1955 In honour <strong>of</strong> <strong>the</strong> founder <strong>of</strong> microchemistry and former pr<strong>of</strong>essor at <strong>the</strong> Graz<br />
University <strong>of</strong> Technology, <strong>the</strong> extended laboratory was called EMICH-Laboratories.<br />
At <strong>the</strong> same time, <strong>the</strong> institute was renamed <strong><strong>Institut</strong>e</strong> <strong>of</strong> Biochemical Technology,<br />
Food Chemistry and Microchemistry.<br />
Lectures and laboratory courses were held in biochemistry, biochemical technology, food<br />
chemistry and food technology, technical microscopy and microchemistry. In addition, <strong>the</strong><br />
institute covered technical microbiology toge<strong>the</strong>r with biological and bacteriological analysis<br />
- with <strong>the</strong> exception <strong>of</strong> pathogenic microorganisms - and a lecture in organic raw materials<br />
sciences.<br />
1970 After <strong>the</strong> decease <strong>of</strong> Pr<strong>of</strong>. GORBACH, Pr<strong>of</strong>. GRUBITSCH was appointed head <strong>of</strong> <strong>the</strong><br />
institute. Towards <strong>the</strong> end <strong>of</strong> <strong>the</strong> sixties, <strong>the</strong> division for water and waste water<br />
disposal headed by Pr<strong>of</strong>. S<strong>TU</strong>NDL was drawn out <strong>of</strong> <strong>the</strong> institute and established as an<br />
independent institute. Pr<strong>of</strong>. SPITZY was nominated pr<strong>of</strong>essor <strong>of</strong> general chemistry,<br />
micro- and radiochemistry. This division was also drawn out <strong>of</strong> <strong>the</strong> mo<strong>the</strong>r institute<br />
and at <strong>the</strong> end <strong>of</strong> <strong>the</strong> sixties moved to a new building.<br />
1973 Division <strong>of</strong> <strong>the</strong> <strong><strong>Institut</strong>e</strong> for Biochemical Technology, Food Technology and<br />
Microchemistry took place. At first, biochemical technology toge<strong>the</strong>r with food<br />
technology formed a new institute now called <strong><strong>Institut</strong>e</strong> <strong>of</strong> Biotechnology and Food<br />
Chemistry headed by Pr<strong>of</strong>. LAFFERTY.<br />
1973 Dr. F. PALTAUF, docent at <strong>the</strong> Karl-Franzens-University Graz, was appointed<br />
pr<strong>of</strong>essor and head <strong>of</strong> <strong>the</strong> newly established <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong>. The interest <strong>of</strong><br />
Pr<strong>of</strong>. PALTAUF in studying biological membranes and lipids laid <strong>the</strong> foundation for<br />
2
<strong>the</strong> future direction <strong>of</strong> research. G. DAUM, S. D. KOHLWEIN, and A. HERMETTER<br />
joined <strong>the</strong> institute. All three young scientists were given <strong>the</strong> chance to work as post<br />
docs in renown laboratories in Switzerland and <strong>the</strong> USA: G. DAUM with <strong>the</strong> groups<br />
<strong>of</strong> G. Schatz (Basel, Switzerland) and R. Schekman (Berkeley, USA), A.<br />
HERMETTER with J. R. Lakowicz (Baltimore, USA) and S. D. KOHLWEIN with S.<br />
A. Henry (New York, USA). Consequently, independent research groups specialized<br />
in cell biology (G. D.), biophysics (A. H.) and molecular biology (S. D. K.) evolved at<br />
<strong>the</strong> institute in Graz, with <strong>the</strong> group <strong>of</strong> Pr<strong>of</strong>. F. PALTAUF still focusing on <strong>the</strong><br />
chemistry and biochemistry <strong>of</strong> lipids.<br />
Teaching was always a major task <strong>of</strong> <strong>the</strong> institute. Lectures, seminars and laboratory courses<br />
in basic biochemistry were complemented by special lectures, seminars, and courses held by<br />
<strong>the</strong> assistants who became docents in 1985 (G. D.), 1987 (A. H.), and 1992 (S. D. K.).<br />
Lectures in food chemistry and technology were held by C. WEBER and H. SALOMON.<br />
Hence <strong>the</strong> institute was renamed <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong> and Food Chemistry.<br />
1990 The institute moved to a new building at Petersgasse 12. The move was accompanied<br />
by <strong>the</strong> expansion <strong>of</strong> individual research groups and <strong>the</strong> acquisition <strong>of</strong> new equipment<br />
essential for <strong>the</strong> participation in novel collaborative efforts at <strong>the</strong> national and<br />
international level. Thus, <strong>the</strong> <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong>, toge<strong>the</strong>r with partner institutes<br />
from <strong>the</strong> Karl-Franzens-University was <strong>the</strong> driving force to establish Graz as a centre<br />
<strong>of</strong> competence in lipid research.<br />
1993 W. PFANNHAUSER was appointed as pr<strong>of</strong>essor <strong>of</strong> food chemistry. Through his own<br />
enthusiasm and engagement and that <strong>of</strong> his collaborators, this new section <strong>of</strong> <strong>the</strong><br />
institute rapidly developed and <strong>of</strong>fered students additional opportunities to receive a<br />
timely education.<br />
2000 The two sections, biochemistry and food chemistry, being independent <strong>of</strong> each o<strong>the</strong>r<br />
with respect to personnel, teaching, and research, were separated into <strong>the</strong> <strong><strong>Institut</strong>e</strong> <strong>of</strong><br />
<strong>Biochemistry</strong> (Head Pr<strong>of</strong>. PALTAUF) and <strong>the</strong> new <strong><strong>Institut</strong>e</strong> <strong>of</strong> Food Chemistry and<br />
Technology (Head Pr<strong>of</strong>. PFANNHAUSER).<br />
2001 After F. PALTAUF’s retirement, in September 2001, G. DAUM was elected head <strong>of</strong><br />
<strong>the</strong> institute. S. D. KOHLWEIN was appointed full pr<strong>of</strong>essor <strong>of</strong> biochemistry at <strong>the</strong><br />
Karl-Franzens University Graz.<br />
2003 P. MACHEROUX was appointed full pr<strong>of</strong>essor <strong>of</strong> biochemistry in September 2003<br />
and head <strong>of</strong> <strong>the</strong> <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong> in January 2004. His research interests<br />
revolve around topics in protein biochemistry and enzymology and shall streng<strong>the</strong>n <strong>the</strong><br />
already existing activities in this area.<br />
2007 K. ATHENSTAEDT, a long-time associate <strong>of</strong> Pr<strong>of</strong>. DAUM, received <strong>the</strong> venia<br />
legendi for biochemistry. Karin is <strong>the</strong> first woman to complete <strong>the</strong> traditional<br />
habilitation at <strong>the</strong> <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong>!<br />
2009 After <strong>the</strong> retirement <strong>of</strong> Pr<strong>of</strong>. PFANNHAUSER in 2008, <strong>the</strong> <strong><strong>Institut</strong>e</strong> <strong>of</strong> Food<br />
Chemistry and Technology was disbanded and <strong>the</strong> research group <strong>of</strong> Pr<strong>of</strong>. M.<br />
MURKOVIC joined <strong>the</strong> <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong> increasing <strong>the</strong> number <strong>of</strong><br />
independent research groups to five.<br />
3
Highlights <strong>of</strong> 2009<br />
In April 2009 <strong>the</strong> restructuring <strong>of</strong> <strong>the</strong> Faculty <strong>of</strong> Technical Chemistry, Chemical Engineering<br />
and Biotechnology resulted in a partial reunification <strong>of</strong> <strong>the</strong> <strong><strong>Institut</strong>e</strong> <strong>of</strong> Food Chemistry and<br />
Technology with <strong>the</strong> <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong>. During this reshaping Michael Murkovic with<br />
his research group "Chemistry <strong>of</strong> Functional Foods” joined <strong>the</strong> <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong>.<br />
The research group <strong>of</strong> Michael Murkovic is mainly interested in<br />
<strong>the</strong> evaluation <strong>of</strong> reaction mechanisms in foods with respect to <strong>the</strong><br />
reactivity <strong>of</strong> antioxidants in relation to <strong>the</strong> formation <strong>of</strong><br />
carcinogenic substance during heating <strong>of</strong> foods. Merging <strong>of</strong> <strong>the</strong><br />
two research units has led to a reinforcement <strong>of</strong> <strong>the</strong> infrastructure<br />
with emphasis on techniques <strong>of</strong> liquid chromatography and <strong>the</strong><br />
identification <strong>of</strong> small molecules.<br />
As in <strong>the</strong> years before, <strong>the</strong> group <strong>of</strong> Peter Macheroux was very active in publishing <strong>the</strong>ir<br />
research results in scientific journals. Among <strong>the</strong> published articles, one was again selected as<br />
best paper <strong>of</strong> <strong>the</strong> week by <strong>the</strong> editors <strong>of</strong> <strong>the</strong> Journal <strong>of</strong> Biological Chemistry (“Hold on to that<br />
flavin” published in July). This publication also marked <strong>the</strong> end <strong>of</strong> a very successful PhD<br />
<strong>the</strong>sis project submitted by Andreas Winkler who left <strong>the</strong> institute and joined <strong>the</strong> Max-Planck-<br />
<strong><strong>Institut</strong>e</strong> <strong>of</strong> Medical Research in Heidelberg, Germany, as an EMBO long-term postdoctoral<br />
fellow. Just before Christmas, Peter Macheroux was awarded <strong>the</strong> Styrian Research Award for<br />
studies on redox-regulated degradation <strong>of</strong> proteins by <strong>the</strong> quinone reductase-proteasome<br />
complex. This project dates back to Peter Macheroux’ early days at <strong>the</strong> institute and was<br />
supported by several outstanding students and postdocs, most notably Sonja Sollner, Andrea<br />
Wagner, Sigrid Deller and Markus Schober.<br />
P. Macheroux (right), Styrian Research Award 2009<br />
4<br />
G. Daum (right), Euro Fed Lipid Conference 2009<br />
As in previous years, research in <strong>the</strong> laboratory <strong>of</strong> Gün<strong>the</strong>r Daum was supported by grants <strong>of</strong><br />
<strong>the</strong> Austrian Science Fund (FWF). In 2009, a new project devoted to “Phosphatidylserine<br />
Decarboxylases <strong>of</strong> <strong>the</strong> Yeast” started with two new graduate students, Martina Gsell and Vid<br />
V. Flis. In 2009, ano<strong>the</strong>r project named ACIB (Austrian Center <strong>of</strong> Industrial Biotechnology)<br />
supported by <strong>the</strong> Austrian Research Promotion Agency (FFG) and industry was approved. In<br />
this joint project Gün<strong>the</strong>r Daum’s group will be involved in applied research devoted to<br />
Pichia pastoris cell biology. Ano<strong>the</strong>r highlight <strong>of</strong> 2009 was <strong>the</strong> Euro Fed Lipid Conference<br />
(European Federation for <strong>the</strong> Science and Technology <strong>of</strong> Lipids) which was held in October
2009 in Graz under <strong>the</strong> chairmanship <strong>of</strong> Gün<strong>the</strong>r Daum. This conference with more than 600<br />
participants was not only an interesting meeting which combined fundamental and applied<br />
research but also an important event for <strong>the</strong> lipid community in Graz. Gün<strong>the</strong>r Daum will also<br />
chair a FEBS workshop devoted to Microbial Lipids which will take place in May 2010 in<br />
Vienna, Austria.<br />
Basic research in Albin Hermetter’s group was performed in three projects focusing on lipid<br />
(patho)physiology. His contribution to <strong>the</strong> GOLD project (speaker R. Zechner, IMB,<br />
University <strong>of</strong> Graz, funded by <strong>the</strong> GEN-AU program <strong>of</strong> <strong>the</strong> BM.W_F) is devoted to <strong>the</strong><br />
functional proteomic analysis <strong>of</strong> (phospho)lipases relevant to lipid-associated disorders. In <strong>the</strong><br />
SFB LIPOTOX (FWF project, speaker R. Zechner) and <strong>the</strong> recently approved<br />
EuroMEMBRANE consortium OXPHOS (speaker P. Kinnunen, University <strong>of</strong> Helsinki), A.<br />
Hermetter studies <strong>the</strong> toxicity <strong>of</strong> oxidized phospholipids in <strong>the</strong> cells <strong>of</strong> <strong>the</strong> arterial wall<br />
(funded by FWF). Research in this field is also supported by <strong>the</strong> PhD program “Molecular<br />
Enzymlogy” (FWF). Applied research in lipid enzymology has been performed in <strong>the</strong> Kplus<br />
center Applied Biocatalysis at <strong>TU</strong> Graz (funding by FFG and industry).<br />
A. Hermetter (left) and his research group<br />
5<br />
K. A<strong>the</strong>nstaedt, an independent group<br />
leader at <strong>the</strong> <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong><br />
At <strong>the</strong> end <strong>of</strong> <strong>the</strong> year 2008 a grant application <strong>of</strong> Karin A<strong>the</strong>nstaedt was approved by<br />
<strong>the</strong> Austrian Science Fund (FWF), which gave her <strong>the</strong> opportunity to continue her work<br />
at our department as an independent researcher. With this project she set <strong>the</strong> cornerstone<br />
to establish her own research group. Karin A<strong>the</strong>nstaedt’s work is devoted to lipid<br />
metabolism in yeast.<br />
In December 2009, Christian Weber, a former Assistant Pr<strong>of</strong>essor at our department, died at<br />
<strong>the</strong> age <strong>of</strong> 80. Christian Weber was a food chemist and has set <strong>the</strong> stage for ongoing research<br />
in this field at our department during his active career. Moreover, he had been President <strong>of</strong> <strong>the</strong><br />
Alumni <strong>TU</strong> Graz 1887 for many years. We will remember him as a true representative <strong>of</strong> our<br />
university and a kind colleague. Our deep sympathies are with his widow.
<strong>Biochemistry</strong> group<br />
Group leader: Peter Macheroux<br />
Secretary: Annemarie Portschy<br />
Postdoctoral Fellow: Ines Waldner-Scott<br />
PhD students: Alexandra Binter, Venugopal Gudipati, Tanja Knaus, Silvia Wallner<br />
Technicians: Eva Maria Pointner (maternity leave), Steve Stipsits, Rosemarie Trenker-El-Toukhy<br />
Alumni 2009: Sonja Sollner (PhD), Andreas Winkler (PhD), Martin Puhl (technician)<br />
General description<br />
The fundamental questions in <strong>the</strong> study <strong>of</strong> enzymes, <strong>the</strong> bio-catalysts <strong>of</strong> all living organisms,<br />
revolve around <strong>the</strong>ir ability to select a substrate (substrate specificity) and subject this substrate to<br />
a predetermined chemical reaction (reaction and regio-specificity). In general, only a few amino<br />
acid residues in <strong>the</strong> "active site" <strong>of</strong> an enzyme are involved in this process and hence provide <strong>the</strong><br />
key to <strong>the</strong> processes taking place during enzyme catalysis. Therefore, <strong>the</strong> focus <strong>of</strong> our research is<br />
to achieve a deeper understanding <strong>of</strong> <strong>the</strong> functional role <strong>of</strong> amino acids in <strong>the</strong> active site <strong>of</strong><br />
enzymes with regard to substrate-recognition and stereo- and regiospecificity <strong>of</strong> <strong>the</strong> chemical<br />
transformation. In addition, we are also interested in substrate-triggered conformational changes<br />
and how enzymes utilize c<strong>of</strong>actors (flavin, nicotinamide) to achieve catalysis. Towards <strong>the</strong>se aims<br />
we employ a multidisciplinary approach encompassing kinetic, <strong>the</strong>rmodynamic, spectroscopic and<br />
structural techniques. In addition, we use site-directed mutagenesis to generate mutant enzymes to<br />
probe <strong>the</strong>ir functional role in <strong>the</strong> mentioned processes. Fur<strong>the</strong>rmore, we collaborate with our<br />
partners in academia and industry to develop inhibitors for enzymes, which can yield important<br />
new insights into enzyme mechanisms and can be useful as potential lead compounds in <strong>the</strong> design<br />
<strong>of</strong> new drugs.<br />
The methods established in our laboratory comprise kinetic (stopped-flow and rapid quench<br />
analysis <strong>of</strong> enzymatic reactions), <strong>the</strong>rmodynamic (iso<strong>the</strong>rmal titration microcalorimetry) and<br />
spectroscopic (fluorescence, circular dichroism and UV/VIS absorbance) methods. We also<br />
frequently use MALDI-TOF and ESI mass spectrometry, protein purification techniques<br />
(chromatography and electrophoresis) and modern molecular biology methods to clone and<br />
express genes <strong>of</strong> interest. A brief description <strong>of</strong> our current research projects is given below.<br />
Berberine bridge enzyme & o<strong>the</strong>r flavin-dependent plant oxidases<br />
Berberine bridge enzyme (BBE) is a central enzyme in <strong>the</strong> biosyn<strong>the</strong>sis <strong>of</strong> berberine, a<br />
pharmaceutically important alkaloid. The enzyme possesses a covalently attached FAD moiety,<br />
which is essential for catalysis. The reaction involves <strong>the</strong> oxidation <strong>of</strong> <strong>the</strong> N-methyl group <strong>of</strong> <strong>the</strong><br />
substrate (S)-reticuline by <strong>the</strong> enzyme-bound flavin and concomitant formation <strong>of</strong> a carbon-carbon<br />
bond (<strong>the</strong> “bridge”). The ultimate acceptor <strong>of</strong> <strong>the</strong> substrate-derived electrons is dioxygen, which<br />
reoxidizes <strong>the</strong> flavin to its resting state:<br />
6
The BBE-catalysed oxidative carbon-carbon bond formation is a new example <strong>of</strong> <strong>the</strong> versatility <strong>of</strong><br />
<strong>the</strong> flavin c<strong>of</strong>actor in biochemical reactions. Our goal is to understand <strong>the</strong> oxidative cyclization<br />
reaction by a biochemical and structural approach.<br />
Recently, we have developed a new expression system for BBE (using cDNA from Eschscholzia<br />
california, gold poppy) in Pichia pastoris, which produces large amounts <strong>of</strong> <strong>the</strong> protein (ca. 500<br />
mg from a 10-L culture). The availability <strong>of</strong> suitable quantities <strong>of</strong> BBE enabled us to crystallize<br />
<strong>the</strong> protein and to solve <strong>the</strong> structure in collaboration with Pr<strong>of</strong>. Karl Gruber at <strong>the</strong> Karl-Franzens<br />
University Graz (see below).<br />
Based on <strong>the</strong> three-dimensional structure <strong>of</strong> BBE, we have performed a site-directed<br />
mutagenesis program to investigate <strong>the</strong> role <strong>of</strong> amino acids present in <strong>the</strong> active site <strong>of</strong> <strong>the</strong><br />
enzyme. In conjunction with o<strong>the</strong>r experiments, this has led to <strong>the</strong> formulation <strong>of</strong> a new<br />
reaction mechanism for <strong>the</strong> enzyme (<strong>the</strong>sis project <strong>of</strong> Andreas Winkler). In collaboration with<br />
Pr<strong>of</strong>. Toni Kutchan at <strong>the</strong> Donald Danforth Plant Science Center in St. Louis, we have<br />
identified o<strong>the</strong>r plant genes that apparently encode flavin-dependent oxidases. These genes are<br />
7
currently expressed in our laboratory in order to characterize <strong>the</strong> role <strong>of</strong> <strong>the</strong> enzymes in<br />
alkaloid biosyn<strong>the</strong>sis (<strong>the</strong>sis project <strong>of</strong> Silvia Wallner).<br />
Dipeptidylpeptidase III<br />
Dipeptidyl-peptidases III (DPPIII; EC 3.4.14.4) are Zn-dependent enzymes with molecular<br />
masses <strong>of</strong> ca. 80-85 kDa that specifically cleave <strong>the</strong> first two amino acids from <strong>the</strong> Nterminus<br />
<strong>of</strong> different length peptides. All known DPPIII sequences contain <strong>the</strong> unique motif<br />
HEXXGH, which enabled <strong>the</strong> recognition <strong>of</strong> <strong>the</strong> dipeptidyl-peptidase III family as a distinct<br />
evolutionary metallopeptidase family (M49). In mammals, DPPIII is associated with<br />
important physiological functions such as pain regulation, and hence <strong>the</strong> enzyme is a potential<br />
drug target. Sigrid Deller and Nina Jajcanin-Jozic have successfully expressed, purified and<br />
characterized <strong>the</strong> recombinant yeast enzyme, and Pravas Baral in Karl Gruber’s laboratory at<br />
<strong>the</strong> Karl-Franzens-University has elucidated <strong>the</strong> crystal structure <strong>of</strong> <strong>the</strong> protein. This work<br />
revealed that yeast DPPIII is <strong>the</strong> first representative <strong>of</strong> a novel protein topology:<br />
We are now focusing on structures with potential substrates or inhibitors bound in <strong>the</strong> active site.<br />
Towards that goal, we are generating inactive mutant proteins to enable cocrystallisation with<br />
peptide substrates. In addition, we have produced recombinant human DPPIII and are in <strong>the</strong><br />
process to determine <strong>the</strong> crystal structure to provide <strong>the</strong> basis for <strong>the</strong> development <strong>of</strong> potentially<br />
useful inhibitors <strong>of</strong> <strong>the</strong> enzyme (Gustavo Arruda’s <strong>the</strong>sis project in Pr<strong>of</strong>. Gruber’s laboratory<br />
supported by Alexandra Binter).<br />
8
Nikkomycin biosyn<strong>the</strong>sis<br />
Nikkomycins are produced by several species <strong>of</strong> Streptomyces and exhibit fungicidal, insecticidal<br />
and acaricidal properties due to <strong>the</strong>ir strong inhibition <strong>of</strong> chitin synthase. Nikkomycins are<br />
promising compounds in <strong>the</strong> cure <strong>of</strong> <strong>the</strong> immunosuppressed, such as AIDS patients, organ<br />
transplant recipients and cancer patients undergoing chemo<strong>the</strong>rapy. Nikkomycin Z (R 1 = uracil &<br />
R 2 = OH, see below) is currently in clinical trial for its antifungal activity. Structurally,<br />
nikkomycins can be classified as peptidyl nucleosides containing two unusual amino acids, i.e.<br />
hydroxypyridylhomothreonine (HPHT) and aminohexuronic acid with an N-glycosidically linked<br />
base:<br />
Although <strong>the</strong> chemical structures <strong>of</strong> nikkomycins have been known since <strong>the</strong> 1970s, only a few<br />
biosyn<strong>the</strong>tic steps have been investigated in detail. The steps leading to <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong><br />
aminohexuronic acid are unclear. Originally, it was hypo<strong>the</strong>sized that <strong>the</strong> aminohexuronic acid<br />
moiety is generated by addition <strong>of</strong> an enolpyruvyl moiety from phosphoenolpyruvate (PEP) to<br />
ei<strong>the</strong>r <strong>the</strong> uridine or <strong>the</strong> 4-formyl-4-imidazolin-2-one analog at <strong>the</strong> 5’-position <strong>of</strong> ribose. This step<br />
is <strong>the</strong>n followed by ra<strong>the</strong>r speculative modifications to yield <strong>the</strong> aminohexuronic acid precursor. In<br />
contrast to this hypo<strong>the</strong>sis, we could recently demonstrate that UMP ra<strong>the</strong>r than uridine serves as<br />
<strong>the</strong> acceptor for <strong>the</strong> enolpyruvyl moiety, a reaction catalyzed by an enzyme encoded by a gene <strong>of</strong><br />
<strong>the</strong> nikkomycin operon termed nikO. Fur<strong>the</strong>rmore, we could demonstrate that it is attached to <strong>the</strong><br />
3’- ra<strong>the</strong>r than <strong>the</strong> 5’-position <strong>of</strong> UMP. These results are very intriguing since none <strong>of</strong> <strong>the</strong><br />
nikkomycins syn<strong>the</strong>sized possess an enolpyruvyl group in this position <strong>of</strong> <strong>the</strong> sugar moiety.<br />
Hence, it must be concluded that <strong>the</strong> resulting 3’-enolpyruvyl-UMP is subject to rearrangement<br />
reactions where <strong>the</strong> enolpyruvyl is detached from its 3’-position and transferred to <strong>the</strong> 5’-position<br />
<strong>of</strong> <strong>the</strong> ensuing aminohexuronic acid moiety. Currently, nothing is known about <strong>the</strong> enzymes<br />
involved in <strong>the</strong>se putative chemical steps. However, <strong>the</strong> genes that are co-transcribed with nikO<br />
have been reported and are designated as nikI, nikJ, nikK, nikL, nikM and nikN. In order to<br />
investigate <strong>the</strong> role <strong>of</strong> <strong>the</strong> encoded proteins in <strong>the</strong> biosyn<strong>the</strong>sis <strong>of</strong> <strong>the</strong> aminohexuronic acid moiety,<br />
<strong>the</strong>se genes will be cloned, expressed and <strong>the</strong> proteins purified for biochemical characterization<br />
(<strong>the</strong>sis project <strong>of</strong> Alexandra Binter).<br />
9
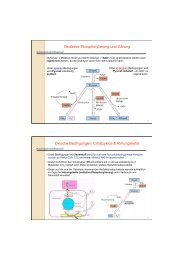
Lot6p – a redox regulated switch <strong>of</strong> <strong>the</strong> proteasome<br />
During our previous studies <strong>of</strong> bacterial quinone reductases, we have also investigated <strong>the</strong><br />
biochemical properties <strong>of</strong> <strong>the</strong> yeast homolog Lot6p. Despite <strong>the</strong> availability <strong>of</strong> a three-dimensional<br />
structure for Lot6p (1T0I), <strong>the</strong> physiological role <strong>of</strong> <strong>the</strong> enzyme was unclear.<br />
Our recent studies have now demonstrated that <strong>the</strong> enzyme rapidly reduces quinones at <strong>the</strong><br />
expense <strong>of</strong> a reduced nicotinamide c<strong>of</strong>actor, ei<strong>the</strong>r NADH or NADPH. In order to fur<strong>the</strong>r<br />
characterize <strong>the</strong> cellular role <strong>of</strong> Lot6p, we have carried out pull-down assays and identified <strong>the</strong> 20S<br />
core particle <strong>of</strong> <strong>the</strong> yeast proteasome as interaction partner. Fur<strong>the</strong>r studies revealed that this<br />
complex recruits Yap4p, a member <strong>of</strong> <strong>the</strong> b-Zip transcription factor family, but only when <strong>the</strong><br />
flavin-c<strong>of</strong>actor <strong>of</strong> Lot6p is in its reduced state. Oxidation <strong>of</strong> <strong>the</strong> flavin leads to dissociation <strong>of</strong> <strong>the</strong><br />
transcription factor and relocalization to <strong>the</strong> nucleus (see scheme below).<br />
reduced<br />
Lot6p<br />
α<br />
β<br />
β<br />
α<br />
A similar system is known from mammalian cells, where a homologous quinone reductase<br />
(NQO1) binds to <strong>the</strong> 20S proteasome and recruits important tumor suppressor proteins such as p53<br />
and p73α. Hence, <strong>the</strong> discovery <strong>of</strong> a homologous protein interaction in yeast provides an<br />
interesting model system to investigate <strong>the</strong> molecular basis for protein complex formation and<br />
regulation <strong>of</strong> proteasomal degradation <strong>of</strong> transcription factors (postdoctoral project <strong>of</strong> Ines<br />
Waldner-Scott; <strong>the</strong>sis project <strong>of</strong> Sonja Sollner and Venugopal Gudipati).<br />
Zn-dependent Alkylsulfatases<br />
Yap4p<br />
Oxidation by<br />
e.g.<br />
quinones<br />
Hydrolysis <strong>of</strong> alkylsulfates is an important pathway for soil and o<strong>the</strong>r bacteria to mobilize sulfur.<br />
Three classes <strong>of</strong> sulfatases have been discovered, and <strong>the</strong> recently elucidated structure <strong>of</strong> SdsA1<br />
from Pseudomonas aeruginosa revealed that <strong>the</strong> enzyme belongs to <strong>the</strong> widely occurring family <strong>of</strong><br />
Zn-dependent ß-lactamases. A detailed characterization <strong>of</strong> <strong>the</strong> enzyme’s stereochemistry by <strong>the</strong><br />
group <strong>of</strong> Pr<strong>of</strong>. Faber at <strong>the</strong> University <strong>of</strong> Graz demonstrated that this enzyme cleaves primary<br />
alklysulfates (preferred substrate is dodecylsulfate) by retention. In o<strong>the</strong>r words, <strong>the</strong> hydroxyl<br />
group attacks <strong>the</strong> sulfur atom in <strong>the</strong> course <strong>of</strong> <strong>the</strong> reaction (see reaction scheme below). Only<br />
recently a novel enzyme activity could be identified in ano<strong>the</strong>r Pseudomonas strain, which leads to<br />
hydrolytic cleavage <strong>of</strong> alkylsulfates via stereoinversion, i. e. <strong>the</strong> hydroxyl group attacks <strong>the</strong> carbon<br />
ra<strong>the</strong>r than <strong>the</strong> sulfur atom <strong>of</strong> <strong>the</strong> substrate (see scheme below).<br />
10<br />
oxidized<br />
Lot6p<br />
Very slow with oxygen O2 nucleus<br />
oxygen<br />
β<br />
α<br />
β<br />
α<br />
Yap4p<br />
Yap4p
HO<br />
R 1<br />
H<br />
R 2<br />
HSO 4 -<br />
Inversion<br />
H<br />
R 1<br />
OH -<br />
O<br />
11<br />
R 2<br />
SO 3 -<br />
OH -<br />
HSO 4 -<br />
Retention<br />
Despite <strong>the</strong> differences in substrate preference and stereochemistry, <strong>the</strong> overall structure <strong>of</strong> this<br />
novel enzyme (termed Pisa1 = Pseudomonas inverting sulfatase) is virtually identical to <strong>the</strong><br />
previously determined structure <strong>of</strong> SdsA1. However, <strong>the</strong> active site <strong>of</strong> Pisa1 features several<br />
conspicuous amino acid exchanges (see figure below: in blue active side residues in SdsA1 and<br />
green those in Pisa1).<br />
Phe/Gly Tyr/His<br />
Ala/Ile<br />
These amino acids are now subject <strong>of</strong> an extensive mutagenesis program to define <strong>the</strong>ir role in<br />
governing substrate preference and <strong>the</strong> stereochemical course <strong>of</strong> <strong>the</strong> reaction. This project is a<br />
close collaboration with Pr<strong>of</strong>s. Faber (biocatalysis) and Wagner (structure determination) from <strong>the</strong><br />
University <strong>of</strong> Graz (<strong>the</strong>sis project <strong>of</strong> Tanja Knaus in our laboratory and Markus Schober in Pr<strong>of</strong>.<br />
Faber’s laboratory).<br />
Doctoral <strong>the</strong>ses completed<br />
Tyr/Ser<br />
Met/Ser<br />
Met/Ser<br />
Tyr/Ser<br />
Leu/Pro<br />
Sonja Sollner: Function and mechanism <strong>of</strong> Lot6p from Saccharomyces cerevisiae, a flavindependent<br />
quinone reductase<br />
Flavin-dependent NAD(P)H:quinone oxidoreductases have been reported from several<br />
mammalian sources. These enzymes have been thought to have a protective effect against<br />
quinone-related oxidative cell damage through <strong>the</strong>ir unique ability to transfer two electrons to a<br />
H<br />
R 1<br />
Ala/Ile<br />
OH<br />
R2
quinone <strong>the</strong>reby catalyzing <strong>the</strong> formation <strong>of</strong> a fully reduced hydroquinone. Interestingly, those<br />
enzymes are found in solid tumours at increased levels and thus can be used to target <strong>the</strong> tumour<br />
cell through bioreductive activation <strong>of</strong> prodrugs. Here <strong>the</strong> identification and characterization <strong>of</strong><br />
Lot6p, a soluble, flavin-dependent quinone reductase from Saccharomyces cerevisiae is described.<br />
It is shown that Lot6p is capable <strong>of</strong> reducing a variety <strong>of</strong> substrates at <strong>the</strong> expense <strong>of</strong> ei<strong>the</strong>r NADH<br />
or NADPH via a ping-pong bi bi reaction mechanism. Recent studies using human quinone<br />
reductases demonstrated that this enzyme binds to <strong>the</strong> 20S proteasome and affects <strong>the</strong> lifetime <strong>of</strong><br />
transcription factors, such as tumour suppressor p53, by inhibiting <strong>the</strong>ir intracellular degradation.<br />
It could be shown that Lot6p is physically associated with <strong>the</strong> yeast proteasome. Fur<strong>the</strong>rmore,<br />
quinone reductase lacking <strong>the</strong> flavin c<strong>of</strong>actor was unable to bind to <strong>the</strong> proteasome indicating that<br />
only <strong>the</strong> holo-protein can form a protein complex. Interaction <strong>of</strong> Lot6p with <strong>the</strong> proteasome is<br />
independent <strong>of</strong> <strong>the</strong> redox state <strong>of</strong> <strong>the</strong> flavin. However, reduction <strong>of</strong> <strong>the</strong> FMN c<strong>of</strong>actor triggers<br />
association <strong>of</strong> <strong>the</strong> complex with Yap4p, a transcription factor involved in <strong>the</strong> oxidative stress<br />
response. Moreover, Yap4p was detected in <strong>the</strong> nucleus <strong>of</strong> quinone stressed yeast cells, but not in<br />
<strong>the</strong> nucleus <strong>of</strong> non-stressed wild-type cells. This finding suggests that stress causes <strong>the</strong> release <strong>of</strong><br />
<strong>the</strong> transcription factor from <strong>the</strong> protein complex resulting in concomittant relocalisation to <strong>the</strong><br />
nucleus. Sequestration <strong>of</strong> a transcription factor by a quinone-reductase-proteasome complex<br />
discovered here is similar to findings in mammalian cells.<br />
Our data also reveal that <strong>the</strong> redox state <strong>of</strong> <strong>the</strong> flavin c<strong>of</strong>actor controls recruitment <strong>of</strong> <strong>the</strong><br />
transcription factor Yap4p to <strong>the</strong> quinone reductase-proteasome complex. This would imply that<br />
stability and localization <strong>of</strong> Yap4p are under direct redox control <strong>of</strong> <strong>the</strong> Lot6p-proteasome<br />
complex. Under reducing conditions (when an excess <strong>of</strong> NAD(P)H is present in <strong>the</strong> cell), Yap4p is<br />
bound to <strong>the</strong> quinone reductase-proteasome system, virtually in a stand-by position. In <strong>the</strong><br />
presence <strong>of</strong> quinones (i.e., when oxidative stress is applied to <strong>the</strong> cell), Yap4p is released from <strong>the</strong><br />
complex and rapidly relocalizes to <strong>the</strong> nucleus where it is now able to switch on stress-related<br />
target genes.<br />
Andreas Winkler: Structure-function studies on berberine bridge enzyme from <strong>the</strong> California<br />
poppy (Eschscholzia californica)<br />
Berberine bridge enzyme catalyzes <strong>the</strong> oxidative conversion <strong>of</strong> (S)-reticuline to (S)-scoulerine by<br />
formation <strong>of</strong> a C-C bond between <strong>the</strong> N-methyl group and <strong>the</strong> phenolic moiety <strong>of</strong> <strong>the</strong> substrate.<br />
This reaction leads to ring closure and is unique to <strong>the</strong> biosyn<strong>the</strong>sis <strong>of</strong> benzophenanthridine<br />
alkaloids with no direct equivalent in organic chemistry. We <strong>the</strong>refore set out to characterize <strong>the</strong><br />
molecular mechanism by means <strong>of</strong> a functional and structural analysis <strong>of</strong> <strong>the</strong> enzyme. The<br />
biochemical characterization <strong>of</strong> <strong>the</strong> flavin c<strong>of</strong>actor revealed that it is covalently linked to <strong>the</strong><br />
protein backbone via two amino acids (His-104 and Cys-166). At <strong>the</strong> time <strong>of</strong> discovery this<br />
unusual bicovalent mode <strong>of</strong> FAD attachment was just recently identified during <strong>the</strong> structural<br />
analysis <strong>of</strong> a distantly related enzyme. We could show that this dual mode <strong>of</strong> c<strong>of</strong>actor linkage<br />
increases <strong>the</strong> redox potential to <strong>the</strong> highest value reported for flavoenzymes so far (+132 mV). The<br />
analysis <strong>of</strong> mutant proteins lacking each <strong>of</strong> <strong>the</strong>se linkages (H104A and C166A) confirmed <strong>the</strong>ir<br />
contribution to <strong>the</strong> elevated redox potential (-100 and -80 mV difference, respectively) but in<br />
addition also demonstrated <strong>the</strong>ir importance for correct positioning <strong>of</strong> <strong>the</strong> substrate in <strong>the</strong> active<br />
site allowing efficient turnover. These conclusions were supported by <strong>the</strong> elucidation <strong>of</strong> <strong>the</strong> crystal<br />
structures <strong>of</strong> wild type BBE as well as those <strong>of</strong> <strong>the</strong> mutant proteins H104A and C166A.<br />
Comparing <strong>the</strong> active site architecture between <strong>the</strong>se structures demonstrated that <strong>the</strong> overall<br />
positioning <strong>of</strong> <strong>the</strong> c<strong>of</strong>actor is only slightly affected by <strong>the</strong> removal <strong>of</strong> <strong>the</strong> covalent linkages, while<br />
surrounding amino acids respond significantly to replacement <strong>of</strong> ei<strong>the</strong>r His-104 or Cys-166.<br />
Especially <strong>the</strong> flexibility <strong>of</strong> Trp-165, which plays an important role during <strong>the</strong> conversion <strong>of</strong><br />
12
substrate to product, was reduced extensively. Its substantial conformational change during<br />
turnover was observed in soaking experiments with (S)-reticuline leading to complex structures<br />
with both substrate and product bound. These structures also allowed <strong>the</strong> identification <strong>of</strong> potential<br />
active site amino acids involved in <strong>the</strong> catalytic mechanism. Determination <strong>of</strong> kinetic rates for<br />
three active site protein variants identified a glutamate residue (Glu-417) to be essential for<br />
c<strong>of</strong>actor reduction and formation <strong>of</strong> <strong>the</strong> berberine bridge. Based on our findings, we proposed a<br />
catalytic mechanism for BBE that entails proton abstraction <strong>of</strong> <strong>the</strong> aromatic hydroxyl group<br />
leading to concerted C-C coupling accompanied by hydride transfer from <strong>the</strong> N-methyl group to<br />
<strong>the</strong> N5 atom <strong>of</strong> <strong>the</strong> FAD c<strong>of</strong>actor.<br />
International cooperations<br />
M. Abramic, Ruder Boskovic <strong><strong>Institut</strong>e</strong> Zagreb, Croatia<br />
F. Hollmann, Delft University <strong>of</strong> Technology, The Ne<strong>the</strong>rlands<br />
M. Groll, Technical University Munich, Germany<br />
T. Kutchan, Donald Danforth Plant Science Center, St. Louis, U.S.A.<br />
S.-H. Liaw, National Yang-Ming University, Taipei, Taiwan<br />
B. A. Palfey, University <strong>of</strong> Michigan, Ann Arbor, U.S.A.<br />
I. Tews, Universität Heidelberg, Germany<br />
Research projects<br />
FWF P19858: “Enzymes <strong>of</strong> nikkomycin biosyn<strong>the</strong>sis”<br />
FWF-Doktoratskolleg “Molecular Enzymology”<br />
WTZ Austria-Croatia “Structure-function relationships in metallopeptidases <strong>of</strong> <strong>the</strong> M49 family”<br />
Publications<br />
1) Sollner, S., Schober, M., Wagner, A., Prem, A., Lorkova, L., Palfey, B. A., Groll, M.,<br />
and Macheroux, P.: Quinone reductase acts as a redox switch <strong>of</strong> <strong>the</strong> 20S yeast<br />
proteasome, EMBO Reports, 2009, 10:65-70.<br />
2) Mueller, N. J., Stueckler, C., Hall, M., Macheroux, P., Faber, K.: Epoxidation <strong>of</strong><br />
conjugated C=C-bonds and sulfur-oxidation <strong>of</strong> thioe<strong>the</strong>rs mediated by NADH:FMNdependent<br />
oxidoreductases, Org. Biomol. Chem., 2009, 7:1115-1119.<br />
3) Wallner, S., Neuwirth, M., Flicker, K., Tews, I., and Macheroux, P.: Dissection <strong>of</strong><br />
contributions from invariant amino acids to complex formation and catalysis in <strong>the</strong><br />
heteromeric pyridoxal-5’-phosphate synthase complex, <strong>Biochemistry</strong>, 2009, 48:1928-<br />
1935.<br />
4) Winkler, A., Motz, K., Riedl, Sabrina, Puhl, M., Macheroux, P., Gruber, K.: Structural<br />
roles <strong>of</strong> bicovalent flavinylation in berberine bridge enzyme, J. Biol. Chem., 2009,<br />
284:19993-20001.<br />
13
5) Neuwirth, M., Strohmeier, M., Windeisen, V., Wallner, S., Deller, S., Rippe, K.,<br />
Sinning, I., Macheroux, P., and Tews, I.: X-ray crystal structure <strong>of</strong> Saccharomyces<br />
cerevisiae Snz1 provides insight into <strong>the</strong> oligomeric nature <strong>of</strong> PLP synthases, FEBS<br />
Lett., 2009, 583:2179-2186.<br />
6) Sollner, S., Macheroux, P.: New roles <strong>of</strong> flavoproteins in molecular cell biology: An<br />
unexpected role for quinone reductases as regulators <strong>of</strong> proteasomal degradation, FEBS<br />
J., 2009, 276:4313-4324.<br />
7) Sollner, S., Durchschlag, M., Fröhlich, K.-U., Macheroux, P.: The redox-sensing<br />
quinone reductase Lot6p acts as an inducer <strong>of</strong> yeast apoptosis, FEMS Yeast Res., 2009,<br />
9:885-891.<br />
8) Winkler, A., Puhl, M., Weber, H., Kutchan, T. M., Gruber, K., Macheroux, P.:<br />
Berberine bridge enzyme catalyzes <strong>the</strong> six-electron oxidation <strong>of</strong> (S)-reticuline to<br />
dehydroscoulerine, Phytochemistry, 2009, 70:1092-1097.<br />
9) Binter, A., Staunig, N., Jelesarov, I., Lohner, K., Palfey, B. A., Deller, S., Gruber, K.,<br />
Macheroux, P.: A single intersubunit salt-bridge affects oligomerization and catalytic<br />
activity in a bacterial quinone reductase, FEBS J., 2009, 276:5263-5274.<br />
10) Breithaupt, C., Kurzbauer, R., Schaller, F., Schaller, A., Huber, R., Macheroux, P., and<br />
Clausen, T.: Structural basis <strong>of</strong> substrate specificity <strong>of</strong> plant 12-oxophytodienoate<br />
reductases, J. Mol. Biol., 2009, 392:1266-1277.<br />
11) Sollner, S., Deller, S., Macheroux, P. and Palfey, B. A.: Mechanism <strong>of</strong> flavin reduction<br />
and oxidation in <strong>the</strong> redox sensing quinone reductase Lot6p from Saccharomyces<br />
cerevisiae, <strong>Biochemistry</strong>, 2009, 48:8636-8643.<br />
12) Grau, M. M., van der Toorn, J., Otten, L. G., Macheroux, P., Taglieber, A., Zilly, F. E.,<br />
Arends, W. C. E., Hollmann, F.: Photoenzymatic reductions <strong>of</strong> C=C double bonds, Adv.<br />
Synth. Catal., 2009, 351:3279-3286.<br />
Award<br />
Styrian Research Award 2009 to Peter Macheroux for his work on “Redox-regulated degradation<br />
<strong>of</strong> proteins by <strong>the</strong> quinone reductase-proteasome complex”.<br />
14
Cell Biology Group<br />
Group leader: Gün<strong>the</strong>r Daum<br />
Graduate students: Tibor Czabany, Melanie Connerth, Sona Rajakumari, Karlheinz Grillitsch,<br />
Miroslava Spanova, Susanne Horvath, Martina Gsell, Vid V. Flis<br />
Diploma student: Sabine Zitzenbacher<br />
Technician: Claudia Hrastnik<br />
Visiting scientists: Sabina Tavares, Fluxome Company, Lyngby, Denmark<br />
General description<br />
The existence <strong>of</strong> functional organelles is <strong>the</strong> basis for regulated processes within a cell. To<br />
sequester organelles from <strong>the</strong>ir environment, membranes are required which not only protect<br />
<strong>the</strong> interior <strong>of</strong> <strong>the</strong> organelles but also govern communication with <strong>the</strong> rest <strong>of</strong> <strong>the</strong> cell.<br />
Therefore, it is very important to understand <strong>the</strong> biogenesis and maintenance <strong>of</strong> biological<br />
membranes. To study this question our laboratory makes use <strong>of</strong> <strong>the</strong> yeast as a well established<br />
experimental system. Our studies are focused on <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> lipids, <strong>the</strong>ir storage and <strong>the</strong>ir<br />
assembly into organelle membranes. We make use <strong>of</strong> <strong>the</strong> advantages <strong>of</strong> this experimental<br />
system to combine biochemical, molecular and cell biological methods addressing problems<br />
<strong>of</strong> lipid metabolism, lipid depot formation and membrane biogenesis.<br />
The majority <strong>of</strong> yeast lipids are syn<strong>the</strong>sized in <strong>the</strong> endoplasmic reticulum (ER) with some<br />
significant contributions <strong>of</strong> mitochondria, <strong>the</strong> Golgi and <strong>the</strong> so-called lipid particles. O<strong>the</strong>r<br />
subcellular fractions are devoid <strong>of</strong> lipid-syn<strong>the</strong>sizing activities. Spatial separation <strong>of</strong> lipid<br />
biosyn<strong>the</strong>tic steps and lack <strong>of</strong> lipid syn<strong>the</strong>sis in several cellular membranes necessitate<br />
efficient transfer <strong>of</strong> lipids from <strong>the</strong>ir site <strong>of</strong> syn<strong>the</strong>sis to <strong>the</strong>ir proper destination(s). The<br />
maintenance <strong>of</strong> organelle lipid pr<strong>of</strong>iles requires strict coordination <strong>of</strong> biosyn<strong>the</strong>tic and<br />
translocation activities.<br />
Specific aspects studied recently in our laboratory are (i) assembly and homeostasis <strong>of</strong><br />
phosphatidylethanolamine in yeast organelle membranes with emphasis on peroxisomes and<br />
<strong>the</strong> plasma membrane, (ii) neutral lipid storage in lipid particles/droplets and mobilization <strong>of</strong><br />
<strong>the</strong>se depots with emphasis on <strong>the</strong> involvement <strong>of</strong> lipases and hydrolases, and (iii)<br />
characterization <strong>of</strong> organelle membranes from <strong>the</strong> industrial yeast Pichia pastoris.<br />
Phosphatidylethanolamine, a key component <strong>of</strong> yeast organelle membranes<br />
Work from our laboratory and from o<strong>the</strong>r groups had shown that phosphatidylethanolamine<br />
(PE), one <strong>of</strong> <strong>the</strong> major phospholipids <strong>of</strong> yeast membranes, is highly important for cellular<br />
function and cell proliferation. PE syn<strong>the</strong>sis in <strong>the</strong> yeast is accomplished by a network <strong>of</strong><br />
reactions including (i) syn<strong>the</strong>sis <strong>of</strong> phosphatidylserine (PS) in <strong>the</strong> endoplasmic reticulum,<br />
(ii) decarboxylation <strong>of</strong> PS by mitochondrial phosphatidylserine decarboxylase 1 (Psd1p) or<br />
(iii) Psd2p in a Golgi/vacuolar compartment, (iv) <strong>the</strong> CDP-ethanolamine pathway (Kennedy<br />
pathway) in <strong>the</strong> endoplasmic reticulum, and (v) <strong>the</strong> lysophospholipid acylation route<br />
catalyzed by Ale1p. To obtain more insight into biosyn<strong>the</strong>sis, assembly and homeostasis <strong>of</strong><br />
PE single and multiple mutants bearing defects in <strong>the</strong> respective pathways can be used.<br />
15
Previous investigations in our laboratory were aimed at <strong>the</strong> molecular biological<br />
identification <strong>of</strong> novel components involved in PE homeostasis <strong>of</strong> <strong>the</strong> yeast Saccharomyces<br />
cerevisiae. For this purpose, a number <strong>of</strong> genetic screenings were performed. To obtain a<br />
global view <strong>of</strong> <strong>the</strong> role <strong>of</strong> PE in <strong>the</strong> cell and to study <strong>the</strong> effects <strong>of</strong> an unbalanced PE level we<br />
recently subjected a psd1∆ deletion mutant and <strong>the</strong> corresponding wild type to DNA<br />
microarray analysis and examined genome-wide changes in gene expression caused by a<br />
PSD1 deletion. Comparison <strong>of</strong> <strong>the</strong> gene expression pattern <strong>of</strong> <strong>the</strong> psd1∆ mutant with <strong>the</strong><br />
wild-type led to <strong>the</strong> identification <strong>of</strong> 55 differentially expressed genes. Grouping <strong>of</strong> <strong>the</strong>se<br />
genes into functional categories revealed that PE formation by Psd1p influenced <strong>the</strong><br />
expression <strong>of</strong> genes involved in diverse cellular pathways including transport, carbohydrate<br />
metabolism and stress response. Most <strong>of</strong> <strong>the</strong> proteins identified are present in <strong>the</strong> cytosol<br />
followed by <strong>the</strong> nucleus, cellular membranes, <strong>the</strong> cell wall and mitochondria. Moreover, this<br />
genome-wide analysis identified a number <strong>of</strong> gene products with unknown function which<br />
are currently subjected to detailed molecular analysis addressing PE homeostasis in <strong>the</strong> yeast.<br />
16<br />
Figure 1:<br />
Three-dimensional reconstruction <strong>of</strong><br />
yeast cells grown on oleic acid.<br />
Transmission electron micrographs <strong>of</strong><br />
ultrathin sections (A, C; bar = 1µm)<br />
and corresponding 3D reconstructions<br />
<strong>of</strong> serial sections (B, D) <strong>of</strong> a<br />
chemically fixed wild type cell are<br />
shown. Cells shown in A and B were<br />
grown on YPD, and cells shown in C<br />
and D were grown on oleic acid to<br />
induce formation <strong>of</strong> peroxisomes. CW<br />
cell wall; ER endoplasmic reticulum;<br />
LP lipid particle; M mitochondrion; N<br />
nucleus; V vacuole; PX peroxisomes.<br />
By courtesy <strong>of</strong> G. Zellnig, KFUG.<br />
To understand cellular PE homeostasis in some more detail, we performed experiments<br />
defining traffic routes <strong>of</strong> PE within <strong>the</strong> yeast cell. In recent studies, we investigated<br />
peroxisomes and <strong>the</strong> plasma membrane as destinations for PE distribution. These two<br />
membranes have in common <strong>the</strong>ir lack <strong>of</strong> capacity to syn<strong>the</strong>size PE. We employed yeast<br />
mutants bearing defects in <strong>the</strong> different pathways <strong>of</strong> PE syn<strong>the</strong>sis and demonstrated that PE<br />
formed though all four pathways can be supplied to peroxisomes and to <strong>the</strong> plasma<br />
membrane. The fatty acid composition <strong>of</strong> peroxisomal and plasma membrane phospholipids<br />
was mostly influenced by <strong>the</strong> available pool <strong>of</strong> total species syn<strong>the</strong>sized, although a certain<br />
balancing effect was observed regarding <strong>the</strong> assembly <strong>of</strong> PE species into <strong>the</strong> two target<br />
compartments. We assume that <strong>the</strong> phospholipid composition <strong>of</strong> peroxisomes and <strong>the</strong> plasma<br />
membrane is mainly affected by <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> <strong>the</strong> respective components and subject to<br />
equilibrium, and to a lesser extent affected by specific transport and assembly processes.
Organelle association and membrane contact between organelles (Fig. 1) may be a relevant<br />
mechanism for lipid transport between organelles, but components governing this process<br />
and related events have to be studied in future investigations.<br />
Growth <strong>of</strong> yeast on oleic acid not only induces formation <strong>of</strong> peroxisomes, but also leads to an<br />
intense syn<strong>the</strong>sis <strong>of</strong> triacylglycerols which are stored in lipid particles (see below). The link<br />
between neutral lipid metabolism and peroxisome proliferation is subject to current<br />
investigations with emphasis on <strong>the</strong> role <strong>of</strong> enzymes involved. These studied are related to<br />
<strong>the</strong> possible lipotoxic effect <strong>of</strong> fatty acids in yeast. Most recently we also studied <strong>the</strong> link<br />
between PE metabolism and neutral lipid storage. For this purpose we analyzed lipids from<br />
strains bearing defects in PE syn<strong>the</strong>sis. These analyses showed that a mutant bearing a defect<br />
in <strong>the</strong> CDP-ethanolamine pathway had a decreased level <strong>of</strong> triacylglycerols (TAG). The<br />
molecular reason for this finding is currently under investigation.<br />
Neutral lipid storage in lipid particles and mobilization<br />
Yeast cells have <strong>the</strong> capacity to store neutral lipids TAG and STE (steryl esters) in subcellular<br />
structures named lipid particles/droplets. Upon requirement, TAG and STE can be mobilized<br />
and serve as building blocks for membrane biosyn<strong>the</strong>sis. In an ongoing project <strong>of</strong> our<br />
laboratory, we focused on <strong>the</strong> characterization <strong>of</strong> enzymatic steps which lead to <strong>the</strong><br />
mobilization <strong>of</strong> TAG and STE depots.<br />
In Saccharomyces cerevisiae, <strong>the</strong> three STE hydrolases Tgl1p, Yeh1p and Yeh2p contribute<br />
differently to STE mobilization from <strong>the</strong>ir site <strong>of</strong> storage, <strong>the</strong> lipid particles. In a biochemical<br />
study we investigated enzymatic and cellular properties <strong>of</strong> <strong>the</strong>se three hydrolytic enzymes.<br />
Using <strong>the</strong> respective single, double and triple deletion mutants and strains overexpressing <strong>the</strong><br />
three enzymes, we demonstrated that each STE hydrolase exhibits certain substrate<br />
specificity. Interestingly, disturbance in STE mobilization also affects sterol biosyn<strong>the</strong>sis in a<br />
type <strong>of</strong> feedback regulation. We also showed that sterol intermediates stored in STE and set<br />
free by STE hydrolases are recycled to <strong>the</strong> sterol biosyn<strong>the</strong>tic pathway and converted to <strong>the</strong><br />
final product, ergosterol. This recycling implies that <strong>the</strong> vast majority <strong>of</strong> sterol precursors are<br />
transported from lipid particles to <strong>the</strong> ER where sterol biosyn<strong>the</strong>sis is completed. Ergosterol<br />
formed through this route is <strong>the</strong>n supplied to its subcellular destinations, especially <strong>the</strong> plasma<br />
membrane. Only a minor amount <strong>of</strong> sterol precursors set free from STE are randomly<br />
distributed within <strong>the</strong> cell after cleavage. In conclusion, STE storage and mobilization<br />
although dispensable for yeast viability contribute markedly to sterol homeostasis and<br />
distribution.<br />
A major focus <strong>of</strong> our neutral lipid project was <strong>the</strong> biochemical characterization <strong>of</strong> <strong>the</strong> three<br />
yeast TAG lipases, Tgl3p, Tgl4p and Tgl5p. Previous work from our laboratory had<br />
demonstrated that deletion <strong>of</strong> TGL3 encoding <strong>the</strong> major yeast TAG lipase resulted in<br />
decreased mobilization <strong>of</strong> TAG, a sporulation defect and a changed pattern <strong>of</strong> fatty acids,<br />
especially increased amounts <strong>of</strong> C22:0 and C26:0 very long chain fatty acids in <strong>the</strong> TAG<br />
fraction. To study a possible link between TAG lipolysis and membrane lipid biosyn<strong>the</strong>sis, we<br />
carried out biochemical experiments with wild type and deletion strains bearing defects in<br />
Tgl3p, Tgl4p and Tgl5p. We demonstrated that tgl mutants had a lower level <strong>of</strong> sphingolipids<br />
and glycerophospholipids than wild type. ESI-MS/MS analyses confirmed that TAG<br />
accumulation in <strong>the</strong>se mutant cells resulted in reduced amounts <strong>of</strong> phospholipids and<br />
sphingolipids. In vitro and in vivo experiments revealed that TAG lipolysis markedly affected<br />
17
<strong>the</strong> metabolic flux <strong>of</strong> long chain fatty acids and very long chain fatty acids required for<br />
sphingolipid and glycerophospholipid syn<strong>the</strong>sis. Moreover, activity and expression levels <strong>of</strong><br />
fatty acid elongases, Elo1p and Elo2p, were enhanced as a consequence <strong>of</strong> reduced TAG<br />
lipolysis emphasizing <strong>the</strong> link to sphingolipid formation. Finally, <strong>the</strong> pattern <strong>of</strong><br />
phosphatidylcholine (PC), PE and PS molecular species was altered in tgl deletion strain<br />
underlining <strong>the</strong> important role <strong>of</strong> TAG turnover in maintenance <strong>of</strong> <strong>the</strong> pool size and<br />
remodelling <strong>of</strong> complex membrane lipids. This study shed new light on <strong>the</strong> physiological role<br />
<strong>of</strong> TAG lipases in yeast and in general.<br />
Figure 2: Domains <strong>of</strong> Tgl3p (from Rajakumari et al., 2010, in press)<br />
Even more surprising evidence was obtained when <strong>the</strong> enzymology <strong>of</strong> <strong>the</strong> three TAG lipases<br />
Tgl3p, Tgl4p and Tgl5p was studied in some detail. Motif search analysis indicated that Tgl3p<br />
and Tgl5p did not only contain <strong>the</strong> TAG lipase but also an acyltransferase motif (Figure 2).<br />
Interestingly, lipid analysis revealed that deletion <strong>of</strong> TGL3 resulted in a decrease and<br />
overexpression <strong>of</strong> TGL3 in an increase <strong>of</strong> glycerophospholipids. Similar results were obtained<br />
with TGL5. Therefore, we tested purified Tgl3p and Tgl5p for acyltransferase activity. Indeed,<br />
both enzymes did not only exhibit lipase activity but also catalyzed acylation <strong>of</strong><br />
lysophosphatidylethanolamine and lysophosphatidic acid, respectively. Experiments using<br />
variants <strong>of</strong> Tgl3p created by site-directed mutagenesis clearly demonstrated that <strong>the</strong> two<br />
enzymatic activities act independently <strong>of</strong> each o<strong>the</strong>r. We also showed that Tgl3p is important<br />
for efficient sporulation <strong>of</strong> yeast cells, but ra<strong>the</strong>r through its acyltransferase than lipase<br />
activity. These results demonstrated that yeast Tgl3p and Tgl5p play a dual role in lipid<br />
metabolism contributing to both anabolic and catabolic processes.<br />
In ano<strong>the</strong>r study, we demonstrated that <strong>the</strong> yeast TAG lipase Tgl4p, <strong>the</strong> functional ortholog <strong>of</strong><br />
adipose TAG lipase (ATGL), catalyzes multiple functions in lipid metabolism. An extended<br />
domain and motif search analysis revealed that Tgl4p bears not only a lipase consensus<br />
domain but also a conserved motif for calcium independent phospholipases A2 (PLA2). We<br />
showed that Tgl4p exhibits TAG lipase, STE hydrolase and PLA2 activities, but also<br />
catalyzes acyl-CoA dependent acylation <strong>of</strong> lysophosphatidic acid (LPA) to phosphatidic acid.<br />
Heterologous overexpression <strong>of</strong> Tgl4p in Pichia pastoris increased total phospholipid and<br />
specifically phosphatidic acid syn<strong>the</strong>sis. Moreover, deletion <strong>of</strong> TGL4 in Saccharomyces<br />
cerevisiae showed an altered pattern <strong>of</strong> PC and phosphatidic acid molecular species.<br />
Altoge<strong>the</strong>r, our data suggested that yeast Tgl4p functions as hydrolytic enzyme in lipid<br />
degradation, but also contributes to fatty acid channelling and phospholipid remodelling.<br />
Several years ago we identified through a mass spectrometric approach <strong>the</strong> major lipid<br />
particle proteins <strong>of</strong> Saccharomyces cerevisiae. This approach was a milestone in <strong>the</strong> field<br />
because it identified a number <strong>of</strong> new gene products and <strong>the</strong>ir function and provided valuable<br />
hints for processes associated with lipid particles. We realized, however, that this study was<br />
18
incomplete and did not provide <strong>the</strong> complete picture <strong>of</strong> <strong>the</strong> yeast lipid particle proteome. For<br />
this reason, a more precise lipid particle proteome analysis was initiated in collaboration with<br />
M. Karas from <strong>the</strong> <strong><strong>Institut</strong>e</strong> <strong>of</strong> Pharmaceutical Chemistry, Johann Wolfgang Goe<strong>the</strong><br />
University, Frankfurt, Germany. This proteome study was combined with a lipidomics<br />
investigation that was performed in collaboration with H. Köfeler from <strong>the</strong> Center for Medical<br />
Research, Medical University <strong>of</strong> Graz, Austria. In this study, we compared lipid particle<br />
components from cells which were grown on glucose or on oleic acid. This approach<br />
identified a number <strong>of</strong> lipid particle proteins that were already known but also some novel<br />
polypeptide candidates. Detailed investigations <strong>of</strong> <strong>the</strong>se proteins are currently in progress. We<br />
also realized through this approach that <strong>the</strong>re were some differences in <strong>the</strong> lipid particle<br />
proteome from cells grown on glucose or oleic acid. Finally, mass spectrometric analyses<br />
revealed marked differences in <strong>the</strong> lipidome <strong>of</strong> lipid particles from cells grown on <strong>the</strong> two<br />
different carbon sources. This study sets <strong>the</strong> stage for fur<strong>the</strong>r investigation <strong>of</strong> protein-lipid<br />
interaction on <strong>the</strong> surface <strong>of</strong> lipid particles and provides basic evidence for <strong>the</strong> coordinated<br />
biosyn<strong>the</strong>sis <strong>of</strong> lipid and protein components from lipid particles.<br />
Ano<strong>the</strong>r important aspect <strong>of</strong> this study was <strong>the</strong> physiological relevance <strong>of</strong> TAG and STE<br />
depot formation. Therefore, attempts were made to understand cell biological consequences <strong>of</strong><br />
dysfunctions in neutral lipid storage. Previously, it has been reported that yeast cells lacking<br />
neutral lipid depots appear to become apoptotic. More recently, we and o<strong>the</strong>rs were able to<br />
demonstrate that lipid depot formation plays an important role especially when yeast cells are<br />
stressed in <strong>the</strong> presence <strong>of</strong> exogenous fatty acids. However, it appears that an adaptation to<br />
this stress situation occurs which helps to overcome <strong>the</strong> deleterious effect <strong>of</strong> this lipotoxic<br />
stress. Changes in <strong>the</strong> lipid pattern caused by cultivation <strong>of</strong> yeast cells on oleic acid are<br />
currently investigated in some detail.<br />
In a previous study we had observed that variation <strong>of</strong> <strong>the</strong> yeast lipid particle composition by<br />
deletion <strong>of</strong> ei<strong>the</strong>r TAG or STE syn<strong>the</strong>sizing enzymes led to changes in <strong>the</strong> protein pattern on<br />
<strong>the</strong> lipid particle surface. In particular we realized that Erg1p, which is present at large<br />
amounts on lipid particles <strong>of</strong> wild type cells, loses stability in strains missing STE. During an<br />
ongoing project we performed stability and topology studies with different variants and<br />
constructs <strong>of</strong> Erg1p to elucidate embedding <strong>of</strong> this protein in <strong>the</strong> lipid particle surface<br />
membrane. This problem is related to <strong>the</strong> general question how lipid particle proteins are<br />
anchored in <strong>the</strong> monolayer membrane <strong>of</strong> <strong>the</strong> particle and/or interact with <strong>the</strong> hydrophobic core<br />
<strong>of</strong> <strong>the</strong> compartment. Although <strong>the</strong> evidence obtained so far is only preliminary we believe that<br />
<strong>the</strong> lipid status <strong>of</strong> lipid particles has a direct influence on <strong>the</strong> protein equipment <strong>of</strong> this<br />
compartment. This link may be related to <strong>the</strong> biogenesis <strong>of</strong> lipid particles.<br />
Ano<strong>the</strong>r potential non-polar storage lipid is squalene. This component belongs to <strong>the</strong> group <strong>of</strong><br />
isoprenoids and is precursor for <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> sterols, steroids and ubiquinons. In <strong>the</strong> yeast,<br />
<strong>the</strong> amount <strong>of</strong> squalene can be increased by varying culture conditions or by genetic<br />
manipulation. As an example, in strains deleted <strong>of</strong> HEM1 squalene accumulates and is stored<br />
mainly in lipid particles. Interestingly, a heme deficient dga1∆lro1∆are1∆are2∆ quadruple<br />
mutant (Qhem1∆) which is devoid <strong>of</strong> <strong>the</strong> classical storage lipids, TAG and SE, accumulates<br />
substantial amounts <strong>of</strong> squalene in cellular membranes, especially in microsomes and <strong>the</strong><br />
plasma membrane. The fact that Qhem1∆ does not form lipid particles suggests that<br />
biogenesis <strong>of</strong> <strong>the</strong>se storage particles cannot be initiated by squalene which is syn<strong>the</strong>sized in<br />
<strong>the</strong> ER similar to TAG and SE. This result also indicates that squalene accumulation, at least<br />
under <strong>the</strong> conditions tested, is not lipotoxic. Finally, our experiments demonstrate that<br />
squalene incorporated into organelle membranes does not compromise cellular function.<br />
19
Pichia pastoris organelles<br />
The yeast Pichia pastoris is an important experimental system for heterologous expression <strong>of</strong><br />
proteins. Never<strong>the</strong>less, surprisingly little is known about organelles <strong>of</strong> this microorganism.<br />
For this reason, we started a systematic biochemical and cell biological study to establish<br />
standardized methods <strong>of</strong> Pichia pastoris organelle isolation and characterization. Recent work<br />
focused on <strong>the</strong> biochemical characterization <strong>of</strong> mitochondrial membranes and <strong>the</strong> plasma<br />
membrane from Pichia pastoris grown on different carbon sources. The major aim <strong>of</strong> this<br />
project was <strong>the</strong> qualitative and quantitative analysis <strong>of</strong> phospholipids, fatty acids and sterols<br />
from both types <strong>of</strong> membranes, when Pichia pastoris was grown on different carbon sources.<br />
We also started to study selected lipid syn<strong>the</strong>sizing enzymes from Pichia pastoris with<br />
emphasis on <strong>the</strong> PE biosyn<strong>the</strong>tic machinery. We identified two Pichia pastoris PS<br />
decarboxylases (PSD) encoded by genes homologous to PSD1 and PSD2 from<br />
Saccharomyces cerevisiae (Figure 3). Using Pichia pastoris psd1 and psd2 mutants we<br />
investigated <strong>the</strong> role <strong>of</strong> <strong>the</strong>se two gene products in PE syn<strong>the</strong>sis and <strong>the</strong> effect <strong>of</strong> <strong>the</strong>ir<br />
deletions on cell growth. Mitochondrial Psd1p is <strong>the</strong> major enzyme for PE syn<strong>the</strong>sis in Pichia<br />
pastoris. Deletion <strong>of</strong> PSD1 caused a loss <strong>of</strong> PSD activity in mitochondria and a severe growth<br />
defect on minimal media which was partially cured by supplementation with ethanolamine.<br />
This result indicated that <strong>the</strong> CDP-ethanolamine but not <strong>the</strong> Psd2p pathway provided<br />
sufficient PE for cell viability in a psd1 background. Under <strong>the</strong>se conditions, however,<br />
cellular and mitochondrial PE levels were dramatically reduced. Fatty acid analysis from wild<br />
type, psd1 and psd2 demonstrated <strong>the</strong> selectivity <strong>of</strong> both PSDs in vivo for <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong><br />
unsaturated PE species. Short chain saturated (16:0) PE species were preferentially imported<br />
into mitochondria irrespective <strong>of</strong> psd1 or psd2 deletions. Thus, biosyn<strong>the</strong>sis and subcellular<br />
distribution contribute to PE homeostasis in mitochondria <strong>of</strong> Pichia pastoris.<br />
.<br />
Figure 3:<br />
Phylogenetic tree <strong>of</strong> PSD sequences from different organisms. The tree was constructed using<br />
<strong>the</strong> neighbor joining (NJ) method <strong>of</strong> <strong>the</strong> CLUSTALW program.<br />
20
Diploma Thesis completed<br />
Sabine Zitzenbacher: Characterization <strong>of</strong> organelles from <strong>the</strong> yeast Pichia pastoris<br />
Characterizations <strong>of</strong> cell organelles from organisms start with a successful isolation <strong>of</strong> <strong>the</strong><br />
organelle <strong>of</strong> interest. Isolation protocols are available, but <strong>the</strong>y usually have to be optimized.<br />
A problem <strong>of</strong> each organelle preparation is cross-contamination with o<strong>the</strong>r cellular organelles<br />
in <strong>the</strong> isolated organelle fraction. Here we tested <strong>the</strong> quality <strong>of</strong> preparation protocols for<br />
mitochondria and microsomes, plasma membrane and vacuoles, used for <strong>the</strong> strain Pichia<br />
pastoris. Isolated fractions were analysed by Western blot analysis and functional enzyme<br />
assays. Results <strong>of</strong> isolated mitochondrial and microsomal fractions showed a clear enrichment<br />
<strong>of</strong> mitochondria as well as microsomes. In <strong>the</strong> mitochondrial fraction a minor ER<br />
contamination was observed, whereas <strong>the</strong> microsomes fractions were free from crosscontamination.<br />
Analysis <strong>of</strong> <strong>the</strong> isolated plasma membrane fraction showed enrichment <strong>of</strong><br />
plasma membrane without any cross contamination with o<strong>the</strong>r organelles. Both preparation<br />
protocols have proven to be well established for Pichia pastoris. The last protocol<br />
investigated showed an enrichment <strong>of</strong> vacuoles in <strong>the</strong> isolated fraction, but <strong>the</strong> vacuolar<br />
fraction also showed a high contamination with mitochondria. Thus, <strong>the</strong> protocol has to be<br />
modified to get an isolated vacuolar fraction without any or with only little crosscontamination.<br />
In <strong>the</strong> past, different cell organelles, such as peroxisomes, mitochondria,<br />
microsomes and plasma membrane from Pichia pastoris were characterized. The missing<br />
organelle is <strong>the</strong> Golgi apparatus. Here we established an isolation protocol for Golgi isolation<br />
from Pichia pastoris. The obtained Golgi fraction was analysed by Western Blot and<br />
enzymatic assays. Applying this new preparation protocol we showed an enrichment <strong>of</strong> Golgi<br />
in <strong>the</strong> isolated fraction largely free <strong>of</strong> cross-contamination with ER.<br />
Doctoral Thesis completed<br />
Tibor Czabany: Cell biology <strong>of</strong> neutral lipid syn<strong>the</strong>sis in yeast Saccharomyces cerevisiae<br />
Previously, four acyltransferases involved in <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> neutral lipids (NL),<br />
triacylglycerols (TAG) and steryl esters (SE), have been identified. Dga1p and Lro1p esterify<br />
DAG to produce TAG, and Are1p and Are2p are responsible for SE syn<strong>the</strong>sis. After <strong>the</strong> NL<br />
have been syn<strong>the</strong>sized <strong>the</strong>y are assembled in specialized organelles called lipid particles (LP).<br />
The hydrophobic core <strong>of</strong> LP containing TAG and SE is separated from <strong>the</strong> hydrophilic<br />
cytosol by a phospholipid monolayer. The present study is focused on elucidation <strong>of</strong> <strong>the</strong><br />
detailed structure using a set <strong>of</strong> triple mutants (TM) which contain only one <strong>of</strong> <strong>the</strong> four<br />
acyltransferases active. Using <strong>the</strong>se mutants we were able to investigate <strong>the</strong> influence <strong>of</strong><br />
individual enzymes on LP composition and structure. It was demonstrated that <strong>the</strong> four<br />
acyltransferases act independently <strong>of</strong> each o<strong>the</strong>r and are able to syn<strong>the</strong>size individually a<br />
sufficient amount <strong>of</strong> neutral lipids for LP biogenesis. Fur<strong>the</strong>rmore, TM and <strong>the</strong> quadruple<br />
mutant, lacking all four acyltransferases, were also used for studies <strong>of</strong> NL syn<strong>the</strong>sis when<br />
fatty acids were supplied as <strong>the</strong> only carbon source. Under <strong>the</strong>se conditions, fatty acids enter<br />
<strong>the</strong> cell and accumulate at high amounts which leads to disturbed lipid homeostasis. Changes<br />
in lipid metabolism caused by <strong>the</strong> lack <strong>of</strong> individual acyltransferase are described, especially<br />
<strong>the</strong> influence <strong>of</strong> excess fatty acids on neutral lipid syn<strong>the</strong>sis. It was shown that yeast cells<br />
grown on oleic acid upregulated TAG syn<strong>the</strong>sis and down regulated esterification <strong>of</strong> sterols.<br />
Detailed analysis <strong>of</strong> SE synthase revealed that <strong>the</strong> down regulation <strong>of</strong> this activity was not by<br />
transcriptional control but ra<strong>the</strong>r by direct inhibition <strong>of</strong> <strong>the</strong> enzyme in its membrane<br />
21
environment. In summary, this work describes biochemical and cell biological functions <strong>of</strong><br />
neutral lipid syn<strong>the</strong>sizing acyltransferases and <strong>the</strong> link to neutral lipid storage in LP.<br />
International Cooperations<br />
J. Cregg, Keck Graduate <strong><strong>Institut</strong>e</strong> <strong>of</strong> Applied Life Sciences, Claremont, CA, USA<br />
I. Hapala, Slovak Academy <strong>of</strong> Sciences, <strong><strong>Institut</strong>e</strong> <strong>of</strong> Animal <strong>Biochemistry</strong> and Genetics,<br />
Ivanka pri Dunaji, Slovak Republic<br />
I. Feussner, Plant <strong>Biochemistry</strong>, Albrecht-von-Haller-<strong><strong>Institut</strong>e</strong> <strong>of</strong> Plant Sciences, Georg-<br />
August University Göttingen, Germany<br />
R. Erdmann, <strong><strong>Institut</strong>e</strong> <strong>of</strong> Physiological Chemistry, Ruhr-University Bochum, Germany<br />
T. Höfken, <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong>, Christian Albrecht University, Kiel, Germany<br />
M. Karas, <strong><strong>Institut</strong>e</strong> <strong>of</strong> Pharmaceutical Chemistry, Johann Wolfgang Goe<strong>the</strong> University,<br />
Frankfurt, Germany<br />
R. Rajasekharan, Department <strong>of</strong> <strong>Biochemistry</strong>, Indian <strong><strong>Institut</strong>e</strong> <strong>of</strong> Science, Bangalore, India<br />
A. S. Sandelius, Department <strong>of</strong> Plant and Environmental Sciences, University <strong>of</strong> Go<strong>the</strong>nburg,<br />
Sweden<br />
T. Höfken, <strong><strong>Institut</strong>e</strong> <strong>of</strong> <strong>Biochemistry</strong>, Christian Albrecht University, Kiel, Germany<br />
Research Projects<br />
FWF 17321: Phosphatidylethanolamine <strong>of</strong> yeast mitochondria<br />
FWF 21429: Phosphatidylserine decarboxylase<br />
FWF 18857: Neutral lipid storage and mobilization in <strong>the</strong> yeast Saccharomyces cerevisiae<br />
FWF L-178 (Translational Research): Characterization <strong>of</strong> organelles from <strong>the</strong> yeast Pichia<br />
pastoris<br />
FWF PhD Program: Molecular Enzymology<br />
Invited Lectures<br />
1. G. Daum<br />
University <strong>of</strong> Bochum, Germany<br />
Turnover <strong>of</strong> neutral lipids and channeling <strong>of</strong> lipid storage components in <strong>the</strong> yeast<br />
April 2009<br />
2. G. Daum, T. Czabany, A. Wagner, K.H. Grillitsch, M. Connerth and S. Rajakumari<br />
Turnover <strong>of</strong> neutral lipids and channeling <strong>of</strong> lipid storage components in <strong>the</strong> yeast<br />
9th Yeast Lipid Conference, Berlin, Germany, 21-23 May 2009<br />
3. G. Daum, T. Czabany, A. Wagner, K.H. Grillitsch, M. Connerth and S. Rajakumari<br />
Turnover <strong>of</strong> neutral lipids and channeling <strong>of</strong> lipid storage components in <strong>the</strong> yeast<br />
27 th ISSY: Pasteur’s legacy – Yeast for Health and Biotechnologies, Paris, France, 26-29<br />
August 2009<br />
22
Publications<br />
1. Wagner, A., Grillitsch, K., Leitner, E. and Daum, G.<br />
Mobilization <strong>of</strong> steryl esters from lipid particles <strong>of</strong> <strong>the</strong> yeast Saccharomyces cerevisiae.<br />
Biochim. Biophys. Acta 1791 (2009) 118-124<br />
2. Schuiki, I., and Daum, G.<br />
Phosphatidylserine decarboxylases, key enzymes <strong>of</strong> lipid metabolism<br />
IUBMB Life 61 (2009) 151-162<br />
3. Wriessnegger, T., Leitner, E., Belegratis, M. R., Ingolic, E. and Daum, G.<br />
Lipid analysis <strong>of</strong> mitochondrial membranes from <strong>the</strong> yeast Pichia pastoris.<br />
Biochim. Biophys. Acta 1791 (2009) 166-172<br />
4. Rosenberger, S., Connerth, M., Zellnig, G. and Daum, G.<br />
Phosphatidylethanolamine syn<strong>the</strong>sized by three different pathways is supplied to<br />
peroxisomes <strong>of</strong> <strong>the</strong> yeast Saccharomyces cerevisiae.<br />
Biochim. Biophys. Acta 1791 (2009) 379-387<br />
5. Wriessnegger, T, Sunga , A. J., Cregg J. M. and Daum, G.<br />
Identification <strong>of</strong> phosphatidylserine decarboxylases 1 and 2 from Pichia pastoris<br />
FEMS Yeast Res. 9 (2009) 911-922<br />
6. Ghosh, A.K., Chauhan, N., Rajakumari, S., Daum, G. and Rajasekharan, R.<br />
At4g24160, A Soluble Acyl-Coenzyme Dependent Lysophosphatidic Acid<br />
Acyltransferase<br />
Plant Physiol. 151 (2009) 869-881<br />
7. Connerth, M., Grillitsch, K., Köfeler, H. and Daum, G.<br />
Analysis <strong>of</strong> Lipid Particles from Yeast<br />
Methods Mol. Biol. 579 (2009) 359-374<br />
8. Lin, M., Grillitsch, K., Daum, G., Just, U. and Höfken, T.<br />
Modulation <strong>of</strong> sterol homeostasis by <strong>the</strong> Cdc42p effectors Cla4p and Ste20p in <strong>the</strong> yeast<br />
Saccharomyces cerevisiae.<br />
FEBS J. 276 (2009) 7253-7264<br />
9. Rajakumari, S. and Daum, G.<br />
Janus-faced enzymes yeast Tgl3p and Tgl5p catalyze lipase and acyltransferase reactions<br />
Mol. Biol. Cell (2009) in press<br />
10. Schuiki, I., Schnabl, M., Czabany, T., Hrastnik, C. and Daum, G.<br />
Phosphatidylethanolamine syn<strong>the</strong>sized by four different pathways is supplied to <strong>the</strong><br />
plasma membrane <strong>of</strong> <strong>the</strong> yeast Saccharomyces cerevisiae.<br />
Biochim. Biophys. Acta (2009) in press<br />
11. Spanova, M., Czabany, T., Zellnig, G., Leitner, E., Hapala, I. and Daum, G.<br />
Effect <strong>of</strong> lipid particle biogenesis on <strong>the</strong> subcellular distribution <strong>of</strong> squalene in <strong>the</strong> yeast<br />
Saccharomyces cerevisiae.<br />
J. Bio. Chem. (2009) in press<br />
Award<br />
Sona Rajakumari<br />
BBA Young Speaker Award, 50th International Conference on Bioscience <strong>of</strong> Lipids (ICBL),<br />
Regensburg, Germany, September 2009<br />
23
Molecular Biology Group<br />
Karin A<strong>the</strong>nstaedt<br />
Triacylglycerol syn<strong>the</strong>sis in <strong>the</strong> oleaginous yeast Yarrowia lipolytica<br />
The oleaginous yeast Yarrowia lipolytica has an outstanding capacity to accumulate huge<br />
amounts <strong>of</strong> triacylglycerols (TAG). Along with o<strong>the</strong>r neutral lipids, TAG are stored in socalled<br />
lipid particles. The structure <strong>of</strong> <strong>the</strong>se cell compartments is ra<strong>the</strong>r simple. Mainly TAG<br />
and steryl esters are forming a hydrophobic core which is surrounded by a phospholipid<br />
monolayer with few proteins embedded. By growing Yarrowia lipolytica cells in media<br />
containing, e.g., industrial fats or glycerol as a carbon source, <strong>the</strong> amount <strong>of</strong> TAG can be<br />
increased up to 40% <strong>of</strong> cell dry weight. This ability leads to its application in biotechnological<br />
processes such as single cell oil production or production <strong>of</strong> nutrients enriched in essential<br />
fatty acids which can serve as nutritional complements. However, Yarrowia lipolytica may<br />
also serve as a model organism to study lipid turnover in adipocytes, since not only <strong>the</strong> ability<br />
to store excessive amounts <strong>of</strong> TAG in lipid particles but also <strong>the</strong> composition <strong>of</strong> this cell<br />
compartment resembles adipocytes <strong>of</strong> higher eukaryotes. Despite <strong>the</strong>se potentials <strong>of</strong> Yarrowia<br />
lipolytica information about TAG (lipid) metabolism in this yeast is ra<strong>the</strong>r limited. Thus, we<br />
started to investigate <strong>the</strong> proteome <strong>of</strong> Yarrowia lipolytica for proteins involved in TAG<br />
syn<strong>the</strong>sis. Homology searches with TAG synthases <strong>of</strong> o<strong>the</strong>r eukaryotes as queries highlighted<br />
two candidate gene-products <strong>of</strong> <strong>the</strong> oleaginous yeast potentially catalyzing <strong>the</strong> formation <strong>of</strong><br />
TAG. A decreased amount <strong>of</strong> TAG in mutant cells defective in <strong>the</strong>se candidate genes already<br />
pinpointed to a function <strong>of</strong> <strong>the</strong>se polypeptides in TAG formation. To investigate whe<strong>the</strong>r<br />
<strong>the</strong>se candidate genes encode true TAG synthases <strong>the</strong>se genes were heterologously expressed<br />
in cells <strong>of</strong> a Saccharomyces cerevisiae mutant defective in neutral lipid syn<strong>the</strong>sis and as a<br />
consequence lacking lipid particles (Fig. 1).<br />
A B<br />
C D<br />
Figure 1: Restoration <strong>of</strong> lipid particle formation upon heterologous expression <strong>of</strong> Yarrowia<br />
lipolytica TAG synthase candidates in mutant cells <strong>of</strong> <strong>the</strong> budding yeast Saccharomyces<br />
cerevisiae lacking lipid particles.<br />
In contrast to <strong>the</strong> negative control (B; mutant + empty plasmid), lipid particles are formed in<br />
mutant cells transformed with plasmids bearing ei<strong>the</strong>r <strong>of</strong> <strong>the</strong> respective candidate genes <strong>of</strong><br />
Yarrowia lipolytica (C, D). Panel A shows lipid particle formation in a wild-type cell <strong>of</strong><br />
Saccharomyces cerevisiae. Lipid particles are indicated by arrows. Size bar: 10 µm.<br />
Fluorescent microscopic inspection and lipid analyses <strong>of</strong> <strong>the</strong> respective mutants clearly<br />
demonstrated that <strong>the</strong>se Yarrowia lipolytica genes encode true TAG synthases. The<br />
24
characteristics <strong>of</strong> <strong>the</strong>se two TAG synthases <strong>of</strong> <strong>the</strong> oleaginous yeast are currently under<br />
investigation.<br />
Is <strong>the</strong> function <strong>of</strong> Nte1p conserved from yeast to man?<br />
Neuropathy target esterase (NTE) was originally identified as <strong>the</strong> target <strong>of</strong><br />
organophosphorous esters (OP) present in many pesticides as well as warfare agents.<br />
Toxifications with OPs are causing delayed neuropathy in man and some o<strong>the</strong>r vertebrates.<br />
The syndrome <strong>of</strong> <strong>the</strong> organophosphorous-induced delayed neuropathy (OPIDN) is<br />
characterized by paralysis <strong>of</strong> <strong>the</strong> lower limbs and degeneration <strong>of</strong> long axons in <strong>the</strong> spinal<br />
cord. These effects are common responses <strong>of</strong> neurons in metabolic disorders (e.g., diabetes),<br />
toxic states and advanced age. Nei<strong>the</strong>r NTE nor its homologs in o<strong>the</strong>r organisms are closely<br />
related to any o<strong>the</strong>r esterase or protease. The importance <strong>of</strong> NTE can be seen by <strong>the</strong> fact that<br />
mice lacking this protein die as early embryos, and inactivation <strong>of</strong> SWS (swiss cheese), <strong>the</strong><br />
homolog <strong>of</strong> NTE in <strong>the</strong> fruit fly Drosophila melanogaster, leads to a progressive degeneration<br />
<strong>of</strong> <strong>the</strong> nervous system, which can be observed as big “holes” in <strong>the</strong> brain <strong>of</strong> <strong>the</strong> fly.<br />
Wild type, 21d sws, 8d<br />
Figure 2: “Holes” in <strong>the</strong> brain <strong>of</strong> a sws-mutant <strong>of</strong> <strong>the</strong> fruit fly Drosophila melanogaster.<br />
Heterologous expression <strong>of</strong> <strong>the</strong> murine NTE can completely substitute SWS in Drosophila,<br />
demonstrating <strong>the</strong> functional conservation <strong>of</strong> this protein. A homolog <strong>of</strong> NTE is also present<br />
in <strong>the</strong> yeast Saccharomyces cerevisiae encoded by NTE1. In contrast to higher eukaryotes,<br />
deletion <strong>of</strong> this protein in <strong>the</strong> model organism yeast does not result in a phenotype under<br />
several standard conditions tested. However, due to <strong>the</strong> presence <strong>of</strong> this polypeptide in a<br />
single cell organism, NTE has to be involved in a more general context as in development,<br />
neural survival and brain integrity. All efforts to identify <strong>the</strong> biological function, substrate,<br />
and interaction partners <strong>of</strong> NTE in higher eukaryotes have failed so far. In contrast, Nte1p <strong>of</strong><br />
<strong>the</strong> yeast has been reported to exhibit phospholipase B activity, thus indicating a similar<br />
function <strong>of</strong> <strong>the</strong> respective counterparts <strong>of</strong> higher eukaryotes. The question arises whe<strong>the</strong>r<br />
Nte1p <strong>of</strong> Saccharomyces cerevisiae is a true member <strong>of</strong> <strong>the</strong> NTE protein family and thus<br />
suitable as a model for unraveling <strong>the</strong> biological function <strong>of</strong> <strong>the</strong> NTE protein family. In vitro,<br />
<strong>the</strong> functional conservation between NTE <strong>of</strong> higher eukaryotes and Nte1p <strong>of</strong> <strong>the</strong> yeast has<br />
already been demonstrated. But is this also true in vivo? For answering this question, I<br />
expressed NTE1 <strong>of</strong> <strong>the</strong> yeast heterologously in <strong>the</strong> fruit fly Drosophila melanogaster. A<br />
functional compensation would be indicated by reversion <strong>of</strong> <strong>the</strong> phenotype <strong>of</strong> an sws mutant<br />
25
fly, i.e., observable as a decrease in <strong>the</strong> size and/or number <strong>of</strong> “holes” in <strong>the</strong> brain <strong>of</strong><br />
Drosophila. Several different mutant lines were constructed expressing <strong>the</strong> yeast NTE1 to<br />
different extent and in different tissues. Subsequently, <strong>the</strong> brains <strong>of</strong> <strong>the</strong>se mutants were<br />
analyzed by fluorescent microscopy for <strong>the</strong> presence <strong>of</strong> “holes”. Any result from full<br />
reversion <strong>of</strong> <strong>the</strong> phenotype to no effect has been considered, however, <strong>the</strong> phenotype was even<br />
worse in <strong>the</strong> transfectants, showing a higher number <strong>of</strong> “holes”. In addition, <strong>the</strong> life span <strong>of</strong><br />
<strong>the</strong> sws-mutants expressing NTE1 <strong>of</strong> yeast was significantly reduced compared to control<br />
flies. These results indicate that <strong>the</strong>re is no functional conservation among <strong>the</strong> NTE protein<br />
family in vivo. However, investigations what caused this ra<strong>the</strong>r striking effect provided some<br />
evidence that Nte1p interacts with enzymes <strong>of</strong> <strong>the</strong> lipid metabolism and forms a protein<br />
complex with at least one more polypeptide. Results <strong>of</strong> experiments performed with<br />
Drosophila indicated that this is also true for Sws <strong>of</strong> <strong>the</strong> fruit fly. Fur<strong>the</strong>rmore, mutations in<br />
certain lipid syn<strong>the</strong>sizing pathways led to smaller and fewer holes in <strong>the</strong> brain <strong>of</strong> a sws-mutant<br />
fly, indicating that <strong>the</strong> phenotype <strong>of</strong> <strong>the</strong> sws mutant is caused by major changes in <strong>the</strong> lipid<br />
pattern. Analysis <strong>of</strong> <strong>the</strong> lipid pattern <strong>of</strong> <strong>the</strong> respective mutant flies is currently under<br />
investigation and will reveal which lipid parameters are required for normal development <strong>of</strong><br />
<strong>the</strong> nervous system. This will provide valuable information for potential targets to compensate<br />
effects caused by neurons in metabolic disorders (e.g., diabetes), toxic states and advanced<br />
age.<br />
International Cooperations<br />
T. Chardot and J.-M. Nicaud, <strong><strong>Institut</strong>e</strong> National de la Recherche Agronomique, UMR Chimie<br />
Biologique, Thiverval Grignon, France<br />
D. Kretzschmar, Oregon Health and Sciences University, Portland, Oregon, USA<br />
Publications<br />
1. A<strong>the</strong>nstaedt, K.<br />
Neutral lipids in yeast: syn<strong>the</strong>sis, storage and degradation.<br />
In Microbiology <strong>of</strong> Hydrocarbons, Oils, Lipids, and Derived Compounds. (Ed. by K. N.<br />
Timmis) Springer-Heidelberg, 2010, 471-480<br />
2. A<strong>the</strong>nstaedt, K.<br />
Players in <strong>the</strong> neutral lipid game – proteins involved in neutral lipid metabolism in<br />
yeast.<br />
In Microbiology <strong>of</strong> Hydrocarbons, Oils, Lipids, and Derived Compounds. (Ed. by K. N.<br />
Timmis) Springer-Heidelberg, 2010, 537-546<br />
3. A<strong>the</strong>nstaedt, K.<br />
Isolation and characterization <strong>of</strong> lipid particles <strong>of</strong> yeast.<br />
In Microbiology <strong>of</strong> Hydrocarbons, Oils, Lipids, and Derived Compounds. (Ed. by K. N.<br />
Timmis) Springer-Heidelberg, 2010, 4223-4229<br />
4. A<strong>the</strong>nstaedt, K.<br />
Neutral lipid metabolism in yeast as a template for biomedical research.<br />
In Microbiology <strong>of</strong> Hydrocarbons, Oils, Lipids, and Derived Compounds. (Ed. by K. N.<br />
Timmis) Springer-Heidelberg, 2010, 3381-3382<br />
26
Biophysical chemistry group<br />
Group leader: Albin Hermetter<br />
Post-docs: Heidrun Susani-Etzerodt, Jo-Anne Rasmussen, Heidemarie Ehammer<br />
Graduate students: Maria Morak, Maximilian Schicher, Ute Stemmer, Daniel Koller, Claudia<br />
Ramprecht, Lingaraju Marlingapla Halasiddappa<br />
Diploma student: Bojana Stojcic<br />
Technicians: Elfriede Zenzmaier<br />
General description<br />
Our research deals with <strong>the</strong> role <strong>of</strong> glycero(phospho)lipids and lipid modifying enzymes as<br />
components <strong>of</strong> membranes and lipoproteins, <strong>the</strong>ir function as mediators in cellular<br />
(patho)biochemistry, and <strong>the</strong>ir application as analytical tools in enzyme technology.<br />
Fluorescence spectroscopy is used as a main technique to investigate <strong>the</strong> behaviour <strong>of</strong> <strong>the</strong>se<br />
biomolecules in <strong>the</strong> respective supramolecular systems.<br />
Section 1 <strong>of</strong> <strong>the</strong> following report summarizes our studies on lipid oxidation, <strong>the</strong> effects <strong>of</strong><br />
oxidized lipids on intracellular signalling, and <strong>the</strong> inhibition <strong>of</strong> <strong>the</strong>se processes by syn<strong>the</strong>tic<br />
and natural antioxidants.<br />
Section 2 describes <strong>the</strong> development <strong>of</strong> fluorescence and chip technology for functional<br />
proteomic analysis <strong>of</strong> lipolytic enzymes in microbial, animal and human cells.<br />
1. Lipid oxidation and a<strong>the</strong>rosclerosis – Lipotoxicity<br />
1.1. Interaction <strong>of</strong> oxidized phospholipids with vascular cells<br />
Sphingomyelin<br />
acid<br />
Sphingomyelinase<br />
Ceramide<br />
JNK<br />
p38 MAPK<br />
Caspase 3<br />
Oxidized -<br />
O<br />
Phospholipids<br />
H<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O P<br />
O<br />
O<br />
OH<br />
+<br />
N<br />
O<br />
O<br />
O<br />
O<br />
O P<br />
O<br />
OH<br />
N +<br />
O<br />
ERK<br />
AKT/PKB<br />
NFkB<br />
PROLIFERATION<br />
SURVIVAL<br />
27<br />
OXPHOS-EuroMEMBRANE<br />
Interactions <strong>of</strong> oxidized lipoproteins with<br />
<strong>the</strong> cells <strong>of</strong> <strong>the</strong> arterial wall induce and<br />
influence <strong>the</strong> progress <strong>of</strong> a<strong>the</strong>rosclerosis.<br />
Accumulation <strong>of</strong> foam cells originating<br />
from macrophages and excessive intimal<br />
growth <strong>of</strong> vascular smooth muscle cells<br />
(SMC) alternating with focal massive cell<br />
death are characteristics typical <strong>of</strong> <strong>the</strong> a<strong>the</strong>rosclerotic lesion. These phenomena are largely<br />
mediated by <strong>the</strong> oxidized phospholipid components <strong>of</strong> <strong>the</strong> modified particles that are<br />
generated under <strong>the</strong> conditions <strong>of</strong> oxidative stress. In <strong>the</strong> framework <strong>of</strong> <strong>the</strong> research consortia<br />
LIPOTOX (SFB) and OXPHOS (EuroMEMBRANE), we investigate <strong>the</strong> “Toxicity <strong>of</strong><br />
oxidized phospholipids in macrophages” and “The protein targets and apoptotic signaling <strong>of</strong><br />
oxidized phospholipids”. These studies aim at identifying <strong>the</strong> molecular and cellular effects <strong>of</strong>
an important subfamily <strong>of</strong> <strong>the</strong> oxidized phospholipids containing long hydrocarbon chains in<br />
position sn-1 and short polar acyl residues in position sn-2 <strong>of</strong> <strong>the</strong> glycerol backbone. The<br />
respective compounds trigger an intracellular signaling network including sphingomyelinases,<br />
(MAP) kinases and transcription factors <strong>the</strong>reby inducing proliferation or apoptosis <strong>of</strong><br />
vascular cells. Both phenomena largely depend on <strong>the</strong> specific action <strong>of</strong> sphingolipid<br />
mediators that are acutely formed upon cell stimulation by <strong>the</strong> oxidized lipids. Fluorescence<br />
microscopic studies on labeled lipid analogs revealed that <strong>the</strong> short-chain<br />
phosphatidylcholines are easily transferred from <strong>the</strong> aqueous phase into <strong>the</strong> cell plasma<br />
membrane and eventually spread throughout <strong>the</strong> cells. Under <strong>the</strong>se circumstances, <strong>the</strong> biologi-<br />
cally active compounds are very likely to interfere<br />
directly with various signaling components inside<br />
<strong>the</strong> cells, which finally decide about cell growth or<br />
death. Investigations are being performed on <strong>the</strong><br />
levels <strong>of</strong> <strong>the</strong> lipidome, <strong>the</strong> apparent enzyme<br />
activities, <strong>the</strong> proteome an <strong>the</strong> transcriptome to find<br />
<strong>the</strong> primary molecular targets and <strong>the</strong>ir downstream<br />
elements that are <strong>the</strong> key compnents <strong>of</strong> lipid-induced<br />
cell death.<br />
1.2. Oxidative stress and antioxidants<br />
Antioxidants protect biomolecules against<br />
oxidative stress and thus, may prevent its<br />
pathological consequences. Specific fluorescent<br />
markers have been established for high-throughput<br />
screening <strong>of</strong> lipid and protein oxidation and its<br />
inhibition by natural and syn<strong>the</strong>tic antioxidants.<br />
These methods can also be used for <strong>the</strong><br />
determination <strong>of</strong> antioxidant capacities <strong>of</strong><br />
biological fluids (e.g. serum) and edible oils. A<br />
prominent example is pumpkin seed oil which<br />
contains a variety <strong>of</strong> antioxidants (e.g. tocopherols<br />
and phenolic substances) and o<strong>the</strong>r secondary plant<br />
components that possess useful biological<br />
properties.<br />
2. Functional proteomic analysis <strong>of</strong> lipolytic enzymes<br />
The functional properties <strong>of</strong> lipases and phospholipases are <strong>the</strong> subject <strong>of</strong> our studies in <strong>the</strong><br />
framework <strong>of</strong> two joint research programs (GOLD-Genomics <strong>of</strong> Lipid-associated Disorders at<br />
KFU Graz and <strong>the</strong> Research center Applied Biocatalysis at <strong>TU</strong> Graz). Lipolytic enzymes<br />
catalyze intra- and extracellular lipid degradation. Dysfunctions in lipid metabolism may lead<br />
to various diseases including obesity, diabetes or a<strong>the</strong>rosclerosis. In chemistry, lipases and<br />
28
esterases are important biocatalysts for (stereo)selective reactions on syn<strong>the</strong>tic and natural<br />
substrates leading to defined products for pharmaceutical or agrochemical use.<br />
2.1. Fluorescent suicide inhibitors for in-gel analysis <strong>of</strong> lipases and phospholipases<br />
Fluorescent suicide inhibitors have been developed as activity recognition probes (ARPs) for<br />
qualitative and quantitative analysis <strong>of</strong> active lipolytic enzymes in complex biological<br />
samples (industrial enzyme preparations, serum, cells, tissues). Since inhibitor binding to <strong>the</strong><br />
active sites <strong>of</strong> lipases and esterases is specific and stoichiometric, accurate information can be<br />
obtained about <strong>the</strong> type <strong>of</strong> enzyme and <strong>the</strong> moles <strong>of</strong> active protein (active sites) in<br />
electrophoretically pure or heterogeneous enzyme preparations.<br />
Labelling <strong>of</strong> enzymes with an ARP specific for serine hydrolases<br />
Inactive<br />
Active<br />
Inactive<br />
Inactive<br />
Inactive<br />
Inhibited<br />
BG<br />
BG<br />
RG<br />
RG<br />
NBD<br />
NBD<br />
RG:<br />
Reacti v e p h osphonate gr o up,<br />
irreversibly inhibits <strong>the</strong> catalytic serine<br />
BG:<br />
Binding group: is specifically recognized<br />
by <strong>the</strong> active enzyme<br />
NBD : fluorescent tag λ : 488 nm; λ : 540 nm<br />
29<br />
ex em<br />
Fluorescent inhibitors are currently applied to proteomic analysis <strong>of</strong> <strong>the</strong> lipolytic enzymes in<br />
human and animal cells. These studies are performed in <strong>the</strong> framework <strong>of</strong> <strong>the</strong> joint project<br />
GOLD (Genomics <strong>of</strong> Lipid-associated Disorders/ coordinated by KFU Graz) which aims at<br />
discovering novel genes, processes and pathways that regulate lipid homeostasis in humans,<br />
mice and yeast. This is one <strong>of</strong> <strong>the</strong> projects in <strong>the</strong> field <strong>of</strong> functional genomics (GEN-AU,<br />
GENome research in AUstria) funded by <strong>the</strong> Austrian Federal Ministry for Education,<br />
Science and Culture (bm:bwk).<br />
Green: wt Red: ko Red: wt Green: ko<br />
DABGE Analysis <strong>of</strong><br />
lipases and esterases<br />
in mouse adipose tissue<br />
<strong>of</strong> wt and lipase ko<br />
mice<br />
Differential activity-based gel electrophoresis (DABGE) was developed for comparative<br />
analysis <strong>of</strong> two lipolytic proteomes in one polyacrylamide gel. For this purpose, <strong>the</strong> active
lipases/esterases <strong>of</strong> two different samples are labelled with fluorescent inhibitors that possess<br />
identical substrate analogous structures but carry different cyanine dyes as reporter<br />
fluorophores. After sample mixing and protein separation by 1-D or 2-D PAGE, <strong>the</strong> enzymes<br />
carrying <strong>the</strong> sample-specific colors are detected and quantified. This technique can be used<br />
for <strong>the</strong> determination <strong>of</strong> differences in enzyme patterns, e.g. due to effects <strong>of</strong> genetic<br />
background, environment, metabolic state and disease.<br />
2.2. Protein microarrays – Enzyme chips<br />
In <strong>the</strong> framework <strong>of</strong> <strong>the</strong> Kplus Research Centre<br />
Applied Biocatalysis (<strong>TU</strong> Graz), we have<br />
developed "biochips" representing ordered<br />
microarrays <strong>of</strong> biospecific ligands for <strong>the</strong> detection<br />
and quantification <strong>of</strong> lipolytic enzymes in<br />
biological samples. In addition, microarrays <strong>of</strong><br />
enzymes are generated to determine protein<br />
affinities for substrate analogs. The development<br />
<strong>of</strong> <strong>the</strong>se tools basically requires three steps, namely<br />
specific loading <strong>of</strong> activated supports with<br />
bioaffinity ligands, measurement <strong>of</strong> lipase binding<br />
(e.g. by fluorescence techniques), and application<br />
30<br />
Angewandte<br />
Biokatalyse<br />
Kompetenzzentrum GmbH<br />
<strong>of</strong> <strong>the</strong> bio chips to <strong>the</strong> investigation <strong>of</strong> real analytical problems. These systems can be used<br />
for <strong>the</strong> screening <strong>of</strong> lipases with specific substrate acceptance.<br />
Diploma <strong>the</strong>sis completed:<br />
Bojana Stojcic: Fluorescence imaging <strong>of</strong> oxidized phospholipid uptake into cultured<br />
macrophages.<br />
Plasma lipoproteins are oxidized as a consequence <strong>of</strong> oxidative stress (enzymatic and<br />
nonenzymatic), which in <strong>the</strong> end can cause lipid and protein modifications <strong>of</strong> <strong>the</strong> particles.<br />
Lipid modifications can induce generation <strong>of</strong> oxidized phospholipids (oxPL) primarily from<br />
(poly)unsaturated diacyl- and alk(en)ylacyl glycerophospholipids including<br />
palmitoyloxovaleroyl-PC (POVPC) and palmitoylglutaroyl-PC (PGPC). Fluorescent analogs<br />
<strong>of</strong> POVPC and PGPC labeled with BODIPY (POVPE-BY and PGPE-BY, respectively) were<br />
used as tools for studying uptake and intracellular stability <strong>of</strong> <strong>the</strong>se compounds in cultured<br />
macrophages. The aim <strong>of</strong> this study was to understand in more detail how oxPL are delivered<br />
to <strong>the</strong> cells and to analyze <strong>the</strong>ir metabolic fate inside <strong>the</strong> cells. The emphasis was on <strong>the</strong> role<br />
<strong>of</strong> <strong>the</strong> (supra)molecular carriers delivering oxPL to <strong>the</strong> cells including lipid micelles, albumin<br />
and (mm)LDL. The import <strong>of</strong> <strong>the</strong> fluorescent lipids from <strong>the</strong>se donors into macrophages was<br />
studied using fluorescence microscopy. In addition, we analyzed <strong>the</strong> transfer <strong>of</strong> oxPL between<br />
albumin and LDL and <strong>the</strong> degradation products <strong>of</strong> PGPE-BY as well as <strong>the</strong> complexes <strong>of</strong> <strong>the</strong><br />
chemically reactive POVPE-BY with proteins or lipids. From our results, it can be inferred<br />
that POVPC mainly elicits its biological effects as a conjugate with lipids and/or proteins. In<br />
order to understand <strong>the</strong> biological activites <strong>of</strong> PGPC, we have to take into account that <strong>the</strong>y<br />
are mediated by various compounds including <strong>the</strong> intact oxPL and its metabolites..
Doctoral Theses completed:<br />
Maria Morak: Functional proteomic analysis and comparison <strong>of</strong> lipolytic enzymes in mouse<br />
tissues<br />
Lipases and esterases catalyze <strong>the</strong> degradation <strong>of</strong> carboxylic acid esters including mono-, di-,<br />
and triacylglycerols, and cholesteryl esters. The hydrolysis <strong>of</strong> <strong>the</strong>se substrates is a key process<br />
in energy homeostasis, lipid remodeling and signaling <strong>of</strong> eucaryotes. Disorders <strong>of</strong> lipid<br />
homeostasis may lead to obesity, type 2 diabetes, a<strong>the</strong>rosclerosis, dyslipidaemia and o<strong>the</strong>r<br />
lipid-associated diseases. In order to identify <strong>the</strong> active lipolytic enzymes in complex<br />
proteomes <strong>of</strong> mouse tissues, fluorescent and biotinylated p-nitrophenyl phosphonic acid esters<br />
were used as activity-recognition probes for selective protein tagging followed by<br />
electrophoretic separation and analysis by nanoHPLC-MS/MS. In addition, differential<br />
activity-based gel electrophoresis (DABGE) was developed as a novel technique for <strong>the</strong><br />
comparison <strong>of</strong> lipolytic enzymes from two different biological samples in one gel. This<br />
method was applied to compare <strong>the</strong> lipolytic proteomes <strong>of</strong> brown and white adipose tissue<br />
from wild-type mice and animals deficient in <strong>the</strong> key enzymes responsible for intracellular<br />
TAG hydrolysis in <strong>the</strong>se organs. We found characteristic lipase patterns in BAT and WAT<br />
that specifically responded to <strong>the</strong> expression <strong>of</strong> ATGL (adipose triglyceride lipase) and HSL<br />
(hormone-sensitive lipase).<br />
Maximilian Schicher: Functional proteomic analysis <strong>of</strong> lipolytic enzymes in cultured murine<br />
and human fat cells<br />
Lipolytic enzymes are responsible for intra- and extracellular degradation <strong>of</strong> acylglycerols and<br />
cholesterol esters. The hydrolysis <strong>of</strong> <strong>the</strong>se compounds is a key event in energy homeostasis<br />
and signaling processes in mammals and humans. Impairment <strong>of</strong> lipid metabolism may lead<br />
to diseases such as insulin resistance, obesity, type 2 diabetes, a<strong>the</strong>rosclerosis and o<strong>the</strong>r lipidassociated<br />
disorders. We used a functional proteomics approach based on fluorescent and<br />
biotinylated p-nitrophenyl phosphonates as activity-based probes to identify active lipolytic<br />
and esterolytic enzymes in subcutaneous and visceral human adipocytes as well as<br />
undifferentiated and differentiated murine fat cells. The labeled proteins were separated by gel<br />
electrophoresis, imaged with a laser scanner and identified by LC-MS/MS after in-gel<br />
digestion. Fur<strong>the</strong>rmore, we compared <strong>the</strong> lipolytic activities <strong>of</strong> subcutaneous and visceral<br />
adipocytes in a single polyacrylamide gel by differential activity-based gel electrophoresis.<br />
We found specific enzyme patterns depending on <strong>the</strong> differentiation state and localization <strong>of</strong><br />
fat cells. The functional analysis <strong>of</strong> overexpressed proteins revealed novel lipase candidates.<br />
International Cooperations:<br />
T. Hianik, Department <strong>of</strong> Biophysics and Chemical Physics, Comenius University, Bratislava,<br />
Slovakia<br />
T. Hornemann, <strong><strong>Institut</strong>e</strong> for Clinical Chemistry, University Hospital Zürich; Zürich,<br />
Switzerland<br />
M. H<strong>of</strong>, Department <strong>of</strong> Biophysical Chemistry, Academy <strong>of</strong> Sciences <strong>of</strong> <strong>the</strong> Czech Republic,<br />
Prague<br />
M. Palmer, Department <strong>of</strong> Chemistry, University <strong>of</strong> Waterloo, Ontario, Canada<br />
D. Russell, Department <strong>of</strong> Microbiology & Immunology, College <strong>of</strong> Veterinary Medicine;<br />
Cornell University, Ithaca, USA<br />
31
R. Yates, Department <strong>of</strong> <strong>Biochemistry</strong> and Molecular Biology, Faculty <strong>of</strong> Medicine,<br />
University <strong>of</strong> Calgary, Calgary, Canada,<br />
N. Faergeman, Department <strong>of</strong> <strong>Biochemistry</strong> and Molecular Biology, University <strong>of</strong> Sou<strong>the</strong>rn<br />
Denmark, Odense, Denmark<br />
Research Projects:<br />
BM.W_F Genomforschung in Österreich (GEN-AU): Exploring <strong>the</strong> lipolytic proteome.<br />
Phospholipases (GOLD 3 project-Genomics <strong>of</strong> Lipid-associated Disorders, coordinated<br />
by KFU Graz).<br />
FWF Special Research Program (SFB) LIPOTOX (coordinated by KFU Graz): Toxicity <strong>of</strong><br />
oxidized phospholipids in macrophages.<br />
FWF Doctoral Program Molecular Enzymology: Functional enzyme analysis.<br />
FWF EUROCORES-EuroMEMBRANE-OXPHOS-Protein targets and apoptotic signaling <strong>of</strong><br />
oxidized phospholipids<br />
TIG Research center Applied Biocatalysis (coordinated by <strong>TU</strong> Graz): Novel lipases with<br />
specific substrate acceptance.<br />
Invited lectures:<br />
1. Signaling <strong>of</strong> oxidized phospholipids in vascular cells. Fluorescent inhibitors for<br />
functional proteomic analysis <strong>of</strong> lipases. Biocrates, Innsbruck, January 21, 2009.<br />
2. Toxicity <strong>of</strong> oxidized phospholipids in macrophages. COST meeting “Free radicals in<br />
Chemical Biology”, Gniezno, Poland, September 11, 2009.<br />
3. The lipolytic proteomes <strong>of</strong> mouse adipose tissue and liver. Cornell University, Ithaca,<br />
NY, USA, September 22, 2009<br />
4. Fluorescent inhibitors for functional proteomic analysis <strong>of</strong> lipolytic enzymes. Euro Fed<br />
Lipid, Graz, Austria, October 20, 2009<br />
Publications:<br />
1. Rhode S, Grurl R, Brameshuber M, Hermetter A, Schütz GJ<br />
Plasma membrane fluidity affects transient immobilization <strong>of</strong> oxidized phospholipids in<br />
endocytotic sites for subsequent uptake.<br />
J. Biol. Chem. 284, 2258-65, 2009<br />
2. Watschinger K, Keller MA, Hermetter A, Golderer G, Werner-Felmayer G, Werner ER<br />
Glyceryl e<strong>the</strong>r monooxygenase resembles aromatic amino acid hydroxylases in metal<br />
ion and tetrahydrobiopterin dependence.<br />
Biol. Chem. 390, 3-10, 2009<br />
3. Yates RM, Hermetter A, Russell DG<br />
Recording phagosome maturation through <strong>the</strong> real-time spectr<strong>of</strong>luorometric<br />
32
measurement <strong>of</strong> hydrolytic activities.<br />
Methods Mol. Biol. 531, 157 -171, 2009<br />
4. Schicher M, Kollroser M, Hermetter A<br />
Mapping <strong>the</strong> lipolytic proteome <strong>of</strong> adipose tissue using fluorescent suicide inhibitors<br />
Methods Mol. Biol. 579, 497-511, 2009.<br />
5. Morak M, Schmidinger H, Krempl P, Rechberger G, Kollroser M, Birner-Gruenberger<br />
R, Hermetter A<br />
Differential activity-based gel electrophoresis for comparative analysis <strong>of</strong> lipolytic and<br />
esterolytic activities.<br />
J. Lipid Res. 50, 1281-92, 2009.<br />
6. Landre JB, Blaess MF, Kohl M, Schlicksbier T, Ruryk A, Kinscherf R, Claus RA,<br />
Hermetter A, Keller M, Bauer M, Deigner HP<br />
Addressable bipartite molecular hook (ABMH): Immobilized hairpin probes with<br />
sensitivity below 50 femtomolar<br />
Anal. Biochem. 2009, Epub ahead <strong>of</strong> print.<br />
33
Chemistry <strong>of</strong> Functional Foods<br />
Group leader: Michael Murkovic<br />
Graduate students: Alam Zeb, Yuliana Reni Swasti<br />
Diploma students: Katrin Julia Greimel, Thomas Binder, Irma Sliumpaite, Bahar Nahimi<br />
Zahabi, Ramune Bobinaite<br />
Technician: Alma Ljubijankic<br />
Honorary Pr<strong>of</strong>essor: Klaus Gün<strong>the</strong>r, Forschungszentrum Jülich, Germany<br />
Project Assistant: Sonja Thanner-Lechner<br />
General description<br />
Antioxidants have different functions depending on <strong>the</strong> location <strong>of</strong> action. Is it <strong>the</strong> protection<br />
<strong>of</strong> biological systems maintaining <strong>the</strong> integrity <strong>of</strong> <strong>the</strong> system or <strong>the</strong> protection <strong>of</strong> foods against<br />
oxidation leading to health threatening substances? The exposure to oxidation products is<br />
ei<strong>the</strong>r described as oxidative stress or <strong>the</strong> oxidized substances have an acute or chronic<br />
toxicity or are carcinogenic. The production <strong>of</strong> healthier and safer foods is <strong>of</strong> primary interest<br />
<strong>of</strong> this research group.<br />
The antioxidants <strong>of</strong> interest are polyphenols including anthocyanins and carotenoids. The<br />
evaluation <strong>of</strong> <strong>the</strong>ir occurrence in foods and <strong>the</strong>ir behavior during processing and cooking is<br />
important especially when <strong>the</strong>se substances are used as food additives. The safety evaluation<br />
<strong>of</strong> <strong>the</strong>se compounds includes <strong>the</strong> evaluation <strong>of</strong> possible degradation products.<br />
Heating <strong>of</strong> foods is a process that is normally done to improve <strong>the</strong> safety and digestibility and<br />
improve <strong>the</strong> sensory attributes like texture, color, and aroma. During <strong>the</strong> heating reactions<br />
occur that lead to <strong>the</strong> degradation <strong>of</strong> nutritive constituents like carbohydrates, proteins, amino<br />
acids and lipids. Some <strong>of</strong> <strong>the</strong> reaction products are contributing to <strong>the</strong> nice aroma, color, and<br />
texture <strong>of</strong> <strong>the</strong> prepared foods and many <strong>of</strong> <strong>the</strong>m are highly toxic and/or carcinogenic.<br />
However, <strong>the</strong>se hazardous compounds occur in ra<strong>the</strong>r low concentrations being normally not<br />
acute toxic. The substances have a very diverse chemical background like heterocyclic<br />
amines, polycondensated aromatic compounds, acrylamide or furan derivatives. The aim <strong>of</strong><br />
<strong>the</strong> research is to investigate <strong>the</strong> reaction mechanisms that lead to <strong>the</strong> formation <strong>of</strong> <strong>the</strong>se<br />
hazardous compounds and establish strategies to mitigate <strong>the</strong> formation and <strong>the</strong>reby reducing<br />
<strong>the</strong> alimentary exposure.<br />
Carotenoids <strong>the</strong>rmal oxidation in triacylglycerols<br />
β-Carotene is one <strong>of</strong> most important and widespread carotenoids present in food. It is an<br />
important source <strong>of</strong> provitamin A. It acts as antioxidant in biological systems including <strong>the</strong><br />
complex system <strong>of</strong> lipids. We studied <strong>the</strong> oxidation <strong>of</strong> β-carotene in model triacylglycerols<br />
system, comparable to most <strong>of</strong> <strong>the</strong> high oleic edible oil. An HPLC electrospray ionization<br />
mass spectrometric method was developed for <strong>the</strong> separation and identification <strong>of</strong><br />
triacylglycerols and its oxidation products (Figure 1). β-Carotene was found to act as<br />
antioxidant at lower temperature like 90 and 100 o C, while oxidation <strong>of</strong> triacyglycerols was<br />
closely correlated with <strong>the</strong> oxidation <strong>of</strong> β-carotene after 8 hours <strong>of</strong> <strong>the</strong>rmal oxidation at<br />
110 o C. The oxidized products <strong>of</strong> β-carotene were epoxides, peroxides and apocarotenals,<br />
separated and analyzed using C30 reversed phase HPLC-DAD and APCI-MS. We found that<br />
110 °C and 120 o C were <strong>the</strong> favorable temperatures for studying <strong>the</strong> oxidation reaction <strong>of</strong><br />
34
carotenoids, while 110 o C, 120 o C and 130 o C were favorable for triacylglycerol oxidation.<br />
Stability <strong>of</strong> triacylglycerols decrease with <strong>the</strong> increased formation <strong>of</strong> <strong>the</strong> oxidation products <strong>of</strong><br />
β-carotene.<br />
Intensity<br />
100<br />
80<br />
60<br />
40<br />
20<br />
Trilinolein<br />
288<br />
263<br />
O<br />
O<br />
O<br />
O<br />
+ [ M + Na]<br />
901<br />
[ M + NH 4]<br />
+<br />
896<br />
+ [ M + H]<br />
879<br />
0<br />
200 300 400 500 600 700 800 900<br />
O<br />
O<br />
m/z<br />
LL +<br />
599<br />
DAGs<br />
Intensity<br />
1-Oleoyl-2-linoleoyl-3-linoleoyl glycerol<br />
100<br />
O<br />
80<br />
60<br />
40<br />
20<br />
Intensity<br />
1-Oleoyl-2-linoleoyl-3-oleoyl glycerol<br />
100<br />
80<br />
60<br />
40<br />
20<br />
+<br />
[ M-<br />
O + H + Li]<br />
874<br />
288<br />
263<br />
+ [ M + H]<br />
883<br />
0<br />
200 300 400 500 600 700 800 900<br />
O<br />
O<br />
O<br />
LL<br />
599<br />
+<br />
+ [ M + Na]<br />
903<br />
[ M + NH4]<br />
+<br />
898<br />
+<br />
[ M-<br />
O + H+<br />
Li]<br />
288<br />
+ 872<br />
[ M + H]<br />
263<br />
881<br />
0<br />
200 300 400 500 600 700 800 900<br />
O<br />
m/z<br />
O<br />
OL<br />
601<br />
+<br />
O<br />
O<br />
O<br />
O<br />
m/z<br />
O<br />
O<br />
OL +<br />
601<br />
OO<br />
603<br />
+<br />
0 5 10 15 20 25 30<br />
Time (min)<br />
35<br />
[ M + Na]<br />
+ 905<br />
[ M + NH4]<br />
+<br />
900<br />
Intensity<br />
4<br />
603<br />
[ M + NH ] +<br />
100 OO 902<br />
+<br />
O<br />
Triolein<br />
80<br />
60<br />
40<br />
O<br />
O<br />
O<br />
20<br />
+<br />
[ M-<br />
O + H + Li]<br />
876<br />
+ [ M + H]<br />
263<br />
883<br />
0<br />
200 300 400 500 600 700 800 900<br />
O<br />
O<br />
m/z<br />
Intensity<br />
1-Oleoyl-2-oleoyl-3-stearoyl glycerol<br />
100<br />
O<br />
80<br />
60<br />
40<br />
20<br />
[ M + Na]<br />
+<br />
907<br />
O<br />
O<br />
O<br />
O<br />
OO<br />
603<br />
+<br />
OS<br />
605<br />
+<br />
[ M + NH4]<br />
+<br />
909<br />
[ M + Na]<br />
+<br />
904<br />
288<br />
+<br />
[ M + H]<br />
887<br />
0<br />
200 300 400 500 600 700 800 900<br />
O<br />
HPLC-MS<br />
Figure 1. Separation and Identification <strong>of</strong> Triacylglycerols using HPLC-ESI-MS.<br />
Diploma Theses completed<br />
Thomas Binder: Effects <strong>of</strong> blanching and enzyme treatment on <strong>the</strong> amount <strong>of</strong> asparagine in<br />
potato slices<br />
The overall aim <strong>of</strong> this work was to find different ways to reduce <strong>the</strong> asparagine content in<br />
raw potato slices. This was done by studying <strong>the</strong> effect <strong>of</strong> blanching and enzyme treatments<br />
on <strong>the</strong> asparagine content in raw and blanched potato slices. A fur<strong>the</strong>r important objective was<br />
to find a good treatment for lowering <strong>the</strong> asparagine content in potato slices which also can be<br />
used in <strong>the</strong> food industry. For this reason <strong>the</strong> cooking value was calculated for <strong>the</strong> different<br />
blanching treatments.<br />
Katrin Julia Greimel: Absolute quantification <strong>of</strong> soy in animal feed<br />
The quantification <strong>of</strong> technically unavoidable contaminations in animal feed (especially soy)<br />
is necessary due to <strong>the</strong> threshold values determined by law. However, it turns out to be a great<br />
challenge for analytical laboratories. In this work, two different methods for <strong>the</strong> absolute<br />
quantification <strong>of</strong> <strong>the</strong> soy percentage in animal feed have been developed. One method for<br />
detection is based on molecular biology <strong>the</strong> real-time polymerase chain reaction (real-time<br />
PCR), <strong>the</strong> o<strong>the</strong>r is a standard method <strong>of</strong> classical analytical chemistry, namely <strong>the</strong> high<br />
performance liquid chromatography (HPLC). Sensitive and specific primers were developed<br />
for <strong>the</strong> PCR-method and were additionally used with suitable hydrolysis probes in a<br />
multiplex-PCR. The optimisation <strong>of</strong> <strong>the</strong> method made it possible to quantify low percentage<br />
soy in animal feed products. The quantification by HPLC was carried out by means <strong>of</strong> <strong>the</strong> two<br />
is<strong>of</strong>lavones daidzein and genistein, that are especially present in soybeans. Both were chosen
ecause <strong>the</strong>y are not present in o<strong>the</strong>r feed ingredients. Using acid hydrolysis for <strong>the</strong> isolation<br />
<strong>of</strong> <strong>the</strong> is<strong>of</strong>lavones, it was possible to determine <strong>the</strong> amount <strong>of</strong> soy in different animal feeds.<br />
The focus <strong>of</strong> this work was set on <strong>the</strong> comparison <strong>of</strong> <strong>the</strong> two methods in terms <strong>of</strong><br />
reproducibility and accuracy.<br />
International Cooperations<br />
R. Venskutonis, <strong><strong>Institut</strong>e</strong> <strong>of</strong> Food Technology, Kaunas University <strong>of</strong> Technology, Lithuania<br />
T. Husoy, National <strong><strong>Institut</strong>e</strong> <strong>of</strong> Public Health, Olso, Norway<br />
H.R. Glatt, Deutsches <strong><strong>Institut</strong>e</strong> <strong>für</strong> Ernährungsforschung, Potsdam Rehbrücke, Germany<br />
E. Lazos, TEJ <strong>of</strong> A<strong>the</strong>ns, Greece<br />
R. Grujic, University <strong>of</strong> East Sarajevo, Bosnia and Herzegovina<br />
E. Habul, University <strong>of</strong> Sarajevo, Bosnia and Herzegovina<br />
E. Winkelhausen, S. Kuzmanova, University Ss Cyril and Methodius, Skopie, FRYM<br />
H. Pinheiro, <strong>Institut</strong>o Superior Tecnico, Lisboa, Portugal<br />
V. Piironen, Department <strong>of</strong> Applied Chemistry and Microbiology, Helsinki, Finland<br />
M.J. Cantalejo, Department <strong>of</strong> Food Technol., Public University <strong>of</strong> Navarre, Pamplona, Spain<br />
Research Projects<br />
European Network <strong>of</strong> Excellence: EuroFIR European Food Information Resource<br />
Invited Lectures<br />
1) M. Murkovic<br />
Alimentary Antioxidants: Relevance in Foods and some Nutritional Aspects<br />
Seminar at JRC-IHCP. 10 Dec. 2009<br />
2) M. Murkovic<br />
Formation <strong>of</strong> hazardous compounds during heating <strong>of</strong> food<br />
Thermally processed foods: possible health implications - Analytical and chemical<br />
aspects related to <strong>the</strong>rmally processed foods. Aveiro, 17 Apr. 2009<br />
Publications<br />
1) Nugroho Prasetyo, E., Kudanga, T., Steiner, W., Murkovic, M., Nyanhongo, G. S.,<br />
Gübitz, G.<br />
Antioxidant activity assay based on laccase-generated radicals<br />
Analytical and bioanalytical chemistry 393 (2009) 2, 679 - 687<br />
2) Murkovic, M.<br />
Europäische Lebensmitteldatenbank: European Food Information Resource Journal <strong>für</strong><br />
Ernährungsmedizin 11 (2009) 3/4, 28 – 29<br />
3) Pricelius, S., Murkovic, M., Souter, P., Gübitz, G.<br />
Substrate specificities <strong>of</strong> glycosidases from Aspergillus species pectinase preparations<br />
on elderberry anthocyanins<br />
Journal <strong>of</strong> agricultural and food chemistry 57 (2009) 3, 1006 - 1012<br />
36
Lectures and Laboratory Courses<br />
Anleitung zu wissenschaftlichen Arbeiten aus Biochemie 2 SE Daum G, Hermetter A,<br />
und Molekularer Biomedizin<br />
Macheroux P<br />
Anleitung zu wissenschaftlichen Arbeiten aus Biochemie 2 SE Daum G, Hermetter A,<br />
und Molekularer Biomedizin<br />
Macheroux P<br />
Bachelorarbeit 1 SE Daum G<br />
Bachelorarbeit 1 SE Daum G<br />
Bioanalytik 2.25 VO Hermetter A, Klimant I<br />
Biochemie I 3,75 VO Macheroux P<br />
Biochemie I LU 5,33 LU <strong>Staff</strong><br />
Biochemie II 1,5 VO Macheroux P<br />
Biochemie II LU 4 LU <strong>Staff</strong><br />
Biophysikalische Chemie 3 PV Laggner P<br />
Biophysikalische Chemie 3 PV Laggner P<br />
Biophysikalische Chemie der Lipide 3 PV Hermetter A<br />
Biophysikalische Chemie der Lipide 3 PV Hermetter A<br />
Chemie und Biowissenschaften 4 VU Hermetter A,<br />
Breinbauer R, Gübitz<br />
G<br />
Chemie und Technologie der Lebensmittel II 2 VO Murkovic M<br />
Chemische Veränderungen in Lebensmitteln 2 PV Murkovic M<br />
Chemische Veränderungen in Lebensmitteln bei der<br />
Verarbeitung<br />
2 SE Murkovic M<br />
Enzymatische und mikrobielle Verfahren in der<br />
Lebensmittelherstellung<br />
2 VO Murkovic M<br />
Fisch- und Fischprodukte 1 VO Murkovic M<br />
Fluoreszenztechnologie 2 VO Hermetter A<br />
Fluoreszenztechnologie 1,5 LU Hermetter A<br />
GL Biochemie (BMT) 2 VO Macheroux P<br />
GL Chemie (BMT) 2 VO Hermetter A<br />
Immunologische Methoden 2 VO Daum G<br />
Immunologische Methoden 2 LU Daum G<br />
Immunologische Methoden 1 VO Daum G<br />
Lebensmittelbiotechnologie 1,3 VO Murkovic M<br />
Lebensmittelbiotechnologie 6 PR Murkovic M<br />
Lebensmittelchemie/Technologie 4 VU Murkovic M<br />
Lebensmittelchemie/Technologie 3 VU Murkovic M<br />
Lebensmitteltechnologisches Praktikum 4 LU Murkovic M<br />
Lebensmitteltechnologisches Praktikum 1 SE Murkovic M<br />
Membran Biophysik 3 PV Lohner K<br />
Membran Biophysik 3 PV Lohner K<br />
Membran-Mimetik 1 VO Lohner K<br />
Molekularbiologie LU 3 LU Waldner-Scott I, Knaus<br />
T<br />
Molekulare Enzymologie 2 VO Gruber K, Macheroux<br />
P, Nidetzky B<br />
Molekulare Enzymologie I 3 PV Macheroux P<br />
37
Molekulare Enzymologie II 3 PV Macheroux P<br />
Molekulare Gastronomie und Lebensmittelverarbeitung II 1 EV Murkovic M<br />
Molekulare Physiologie 2 VO Macheroux P<br />
Physik.Meth.i.d.Biochemie 2 LU Laggner P<br />
Physikalische Methoden in der Biochemie 1 VO Laggner P<br />
Projektlabor Bioanalytik 6 PR Daum G, Hermetter A,<br />
Macheroux P<br />
Projektlabor Bioanalytik 6 PR Daum G, Hermetter A,<br />
Macheroux P<br />
Qualitätssicherung in Pharma-, Lebensmittel- und<br />
Biotechnologie<br />
2 VO Murkovic M<br />
Seminar zu den LU aus Molekularbiologie 1 SE A<strong>the</strong>nstaedt K,<br />
Waldner-Scott I<br />
Seminar zur Masterarbeit aus Biochemie und Molekularer 2 SE Daum G, Hermetter A,<br />
Biomedizin<br />
Macheroux P<br />
Seminar zur Masterarbeit aus Biochemie und Molekularer 2 SE Daum G, Hermetter A,<br />
Biomedizin<br />
Macheroux P<br />
Spezielle Kapitel der Biochemie 1 VO Daum G, Hermetter A,<br />
Macheroux P<br />
Spezielle Kapitel der Lebensmittelchem.- u. technologie I 2 SE Murkovic M<br />
Spezielle Kapitel der Lebensmittelchemie- und techn.II 2 SE Murkovic M<br />
Strukturanalyse in Biophysik u. Materialforschung 2 VO Laggner P<br />
Umwelt- und Lebensmittelbiotechnologie 4 LU Murkovic M<br />
Warenkunde - Lebensmittel 1 SE Murkovic M<br />
Zellbiologie 1 1 SE Daum G<br />
Zellbiologie 2 1 SE Daum G<br />
Zellbiologie der Lipide 3 PV Daum G<br />
Zellbiologie der Lipide 3 PV Daum G<br />
38