Staff Members of the Institute of Biochemistry, TU - Institut für ...
Staff Members of the Institute of Biochemistry, TU - Institut für ...
Staff Members of the Institute of Biochemistry, TU - Institut für ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
currently expressed in our laboratory in order to characterize <strong>the</strong> role <strong>of</strong> <strong>the</strong> enzymes in<br />
alkaloid biosyn<strong>the</strong>sis (<strong>the</strong>sis project <strong>of</strong> Silvia Wallner).<br />
Dipeptidylpeptidase III<br />
Dipeptidyl-peptidases III (DPPIII; EC 3.4.14.4) are Zn-dependent enzymes with molecular<br />
masses <strong>of</strong> ca. 80-85 kDa that specifically cleave <strong>the</strong> first two amino acids from <strong>the</strong> Nterminus<br />
<strong>of</strong> different length peptides. All known DPPIII sequences contain <strong>the</strong> unique motif<br />
HEXXGH, which enabled <strong>the</strong> recognition <strong>of</strong> <strong>the</strong> dipeptidyl-peptidase III family as a distinct<br />
evolutionary metallopeptidase family (M49). In mammals, DPPIII is associated with<br />
important physiological functions such as pain regulation, and hence <strong>the</strong> enzyme is a potential<br />
drug target. Sigrid Deller and Nina Jajcanin-Jozic have successfully expressed, purified and<br />
characterized <strong>the</strong> recombinant yeast enzyme, and Pravas Baral in Karl Gruber’s laboratory at<br />
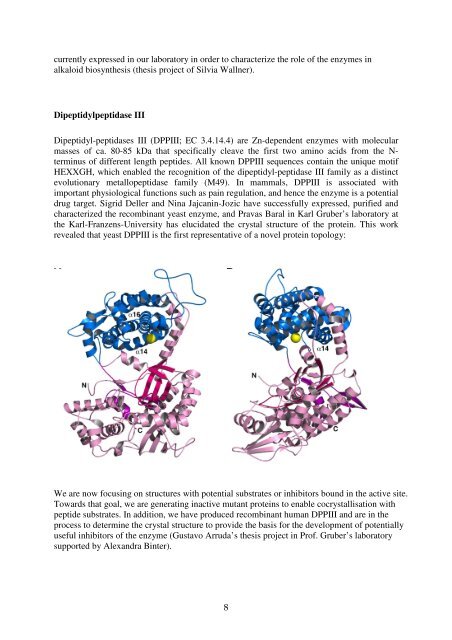
<strong>the</strong> Karl-Franzens-University has elucidated <strong>the</strong> crystal structure <strong>of</strong> <strong>the</strong> protein. This work<br />
revealed that yeast DPPIII is <strong>the</strong> first representative <strong>of</strong> a novel protein topology:<br />
We are now focusing on structures with potential substrates or inhibitors bound in <strong>the</strong> active site.<br />
Towards that goal, we are generating inactive mutant proteins to enable cocrystallisation with<br />
peptide substrates. In addition, we have produced recombinant human DPPIII and are in <strong>the</strong><br />
process to determine <strong>the</strong> crystal structure to provide <strong>the</strong> basis for <strong>the</strong> development <strong>of</strong> potentially<br />
useful inhibitors <strong>of</strong> <strong>the</strong> enzyme (Gustavo Arruda’s <strong>the</strong>sis project in Pr<strong>of</strong>. Gruber’s laboratory<br />
supported by Alexandra Binter).<br />
8