The Lone Star Pharmacist - September 2018
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>The</strong> <strong>Lone</strong> <strong>Star</strong> <strong>Pharmacist</strong> <strong>September</strong> <strong>2018</strong> Page 7<br />
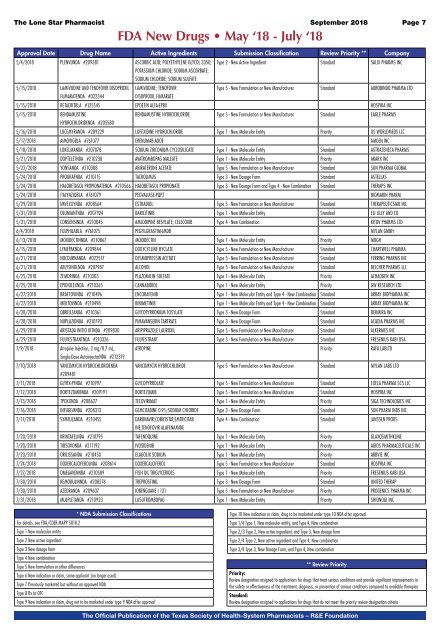
FDA New Drugs • May ‘18 - July ‘18<br />
Approval Date Drug Name Active Ingredients Submission Classification Review Priority ** Company<br />
5/4/<strong>2018</strong> PLENVUNDA #209381 ASCORBIC ACID; POLYETHYLENE GLYCOL 3350; Type 2 - New Active Ingredient Standard SALIX PHARMS INC<br />
POTASSIUM CHLORIDE; SODIUM ASCORBATE;<br />
SODIUM CHLORIDE; SODIUM SULFATE<br />
5/15/<strong>2018</strong> LAMIVUDINE AND TENOFOVIR DISOPROXIL LAMIVUDINE; TENOFOVIR<br />
Type 5 - New Formulation or New Manufacturer Standard AUROBINDO PHARMA LTD<br />
FUMARATENDA #022344<br />
DISOPROXIL FUMARATE<br />
5/15/<strong>2018</strong> RETACRITBLA #125545 EPOETIN ALFA-EPBX HOSPIRA INC<br />
5/15/<strong>2018</strong> BENDAMUSTINE<br />
BENDAMUSTINE HYDROCHLORIDE Type 5 - New Formulation or New Manufacturer Standard EAGLE PHARMS<br />
HYDROCHLORIDENDA #205580<br />
5/16/<strong>2018</strong> LUCEMYRANDA #209229 LOFEXIDINE HYDROCHLORIDE Type 1 - New Molecular Entity Priority US WORLDMEDS LLC<br />
5/17/<strong>2018</strong> AIMOVIGBLA #761077 ERENUMAB-AOOE AMGEN INC<br />
5/18/<strong>2018</strong> LOKELMANDA #207078 SODIUM ZIRCONIUM CYCLOSILICATE Type 1 - New Molecular Entity Standard ASTRAZENECA PHARMS<br />
5/21/<strong>2018</strong> DOPTELETNDA #210238 AVATROMBOPAG MALEATE Type 1 - New Molecular Entity Priority AKARX INC<br />
5/22/<strong>2018</strong> YONSANDA #210308 ABIRATERONE ACETATE Type 5 - New Formulation or New Manufacturer Standard SUN PHARMA GLOBAL<br />
5/24/<strong>2018</strong> PROGRAFNDA #210115 TACROLIMUS Type 3 - New Dosage Form Standard ASTELLAS<br />
5/24/<strong>2018</strong> HALOBETASOL PROPIONATENDA #210566 HALOBETASOL PROPIONATE Type 3 - New Dosage Form and Type 4 - New Combination Standard THERAPS INC<br />
5/24/<strong>2018</strong> PALYNZIQBLA #761079 PEGVALIASE-PQPZ BIOMARIN PHARM<br />
5/29/<strong>2018</strong> IMVEXXYNDA #208564 ESTRADIOL Type 5 - New Formulation or New Manufacturer Standard THERAPEUTICSMD INC<br />
5/31/<strong>2018</strong> OLUMIANTNDA #207924 BARICITINIB Type 1 - New Molecular Entity Standard ELI LILLY AND CO<br />
5/31/<strong>2018</strong> CONSENSINDA #210045 AMLODIPINE BESYLATE; CELECOXIB Type 4 - New Combination Standard KITOV PHARMS LTD<br />
6/4/<strong>2018</strong> FULPHILABLA #761075 PEGFILGRASTIM-JMDB MYLAN GMBH<br />
6/13/<strong>2018</strong> MOXIDECTINNDA #210867 MOXIDECTIN Type 1 - New Molecular Entity Priority MDGH<br />
6/15/<strong>2018</strong> LYMEPAKNDA #209844 DOXYCYCLINE HYCLATE Type 5 - New Formulation or New Manufacturer Standard CHARTWELL PHARMA<br />
6/21/<strong>2018</strong> NOCDURNANDA #022517 DESMOPRESSIN ACETATE Type 5 - New Formulation or New Manufacturer Standard FERRING PHARMS INC<br />
6/21/<strong>2018</strong> ABLYSINOLNDA #207987 ALCOHOL Type 5 - New Formulation or New Manufacturer Standard BELCHER PHARMS LLC<br />
6/25/<strong>2018</strong> ZEMDRINDA #210303 PLAZOMICIN SULFATE Type 1 - New Molecular Entity Priority ACHAOGEN INC<br />
6/25/<strong>2018</strong> EPIDIOLEXNDA #210365 CANNABIDIOL Type 1 - New Molecular Entity Priority GW RESEARCH LTD<br />
6/27/<strong>2018</strong> BRAFTOVINDA #210496 ENCORAFENIB Type 1 - New Molecular Entity and Type 4 - New Combination Standard ARRAY BIOPHARMA INC<br />
6/27/<strong>2018</strong> MEKTOVINDA #210498 BINIMETINIB Type 1 - New Molecular Entity and Type 4 - New Combination Standard ARRAY BIOPHARMA INC<br />
6/28/<strong>2018</strong> QBREXZANDA #210361 GLYCOPYRRONIUM TOSYLATE Type 3 - New Dosage Form Standard DERMIRA INC<br />
6/28/<strong>2018</strong> NUPLAZIDNDA #210793 PIMAVANSERIN TARTRATE Type 3 - New Dosage Form Standard ACADIA PHARMS INC<br />
6/29/<strong>2018</strong> ARISTADA INITIO KITNDA #209830 ARIPIPRAZOLE LAUROXIL Type 5 - New Formulation or New Manufacturer Standard ALKERMES INC<br />
6/29/<strong>2018</strong> FULVESTRANTNDA #210326 FULVESTRANT Type 5 - New Formulation or New Manufacturer Standard FRESENIUS KABI USA<br />
7/9/<strong>2018</strong> Atropine Injection, 2 mg/0.7 mL, ATROPINE Priority RAFA LABLTD<br />
Single-Dose AutoinjectorNDA #212319<br />
7/10/<strong>2018</strong> VANCOMYCIN HYDROCHLORIDENDA VANCOMYCIN HYDROCHLORIDE Type 5 - New Formulation or New Manufacturer Standard MYLAN LABS LTD<br />
#209481<br />
7/11/<strong>2018</strong> GLYRX-PFNDA #210997 GLYCOPYRROLATE Type 5 - New Formulation or New Manufacturer Standard EXELA PHARMA SCS LLC<br />
7/12/<strong>2018</strong> BORTEZOMIBNDA #209191 BORTEZOMIB Type 5 - New Formulation or New Manufacturer Standard HOSPIRA INC<br />
7/13/<strong>2018</strong> TPOXXNDA #208627 TECOVIRIMAT Type 1 - New Molecular Entity Priority SIGA TECHNOLOGIES INC<br />
7/16/<strong>2018</strong> INFUGEMNDA #208313 GEMCITABINE 0.9%;SODIUM CHLORIDE Type 3 - New Dosage Form Standard SUN PHARM INDS INC<br />
7/17/<strong>2018</strong> SYMTUZANDA #210455 DARUNAVIR;COBICISTAT;EMTRICITAB Type 4 - New Combination Standard JANSSEN PRODS<br />
INE;TENOFOVIR ALAFENAMIDE<br />
7/20/<strong>2018</strong> KRINTAFELNDA #210795 TAFENOQUINE Type 1 - New Molecular Entity Priority GLAXOSMITHKLINE<br />
7/20/<strong>2018</strong> TIBSOVONDA #211192 IVOSIDENIB Type 1 - New Molecular Entity Priority AGIOS PHARMACEUTICALS INC<br />
7/23/<strong>2018</strong> ORILISSANDA #210450 ELAGOLIX SODIUM Type 1 - New Molecular Entity Priority ABBVIE INC<br />
7/24/<strong>2018</strong> DOXERCALCIFEROLNDA #208614 DOXERCALCIFEROL Type 5 - New Formulation or New Manufacturer Standard HOSPIRA INC<br />
7/27/<strong>2018</strong> OMEGAVENNDA #210589 FISH OIL TRIGLYCERIDES Type 1 - New Molecular Entity Priority FRESENIUS KABI USA<br />
7/30/<strong>2018</strong> REMODULINNDA #208276 TREPROSTINIL Type 3 - New Dosage Form Standard UNITED THERAP<br />
7/30/<strong>2018</strong> AZEDRANDA #209607 IOBENGUANE I 131 Type 5 - New Formulation or New Manufacturer Priority PROGENICS PHARMA INC<br />
7/31/<strong>2018</strong> MULPLETANDA #210923 LUSUTROMBOPAG Type 1 - New Molecular Entity Priority SHIONOGI INC<br />
* NDA Submission Classifications<br />
For details, see FDA/CDER MAPP 5018.2<br />
Type 1 New molecular entity<br />
Type 2 New active ingredient<br />
Type 3 New dosage form<br />
Type 4 New combination<br />
Type 5 New formulation or other differences<br />
Type 6 New indication or claim, same applicant [no longer used]<br />
Type 7 Previously marketed but without an approved NDA<br />
Type 8 Rx to OTC<br />
Type 9 New indication or claim, drug not to be marketed under type 9 NDA after approval<br />
Type 10 New indication or claim, drug to be marketed under type 10 NDA after approval<br />
Type 1/4 Type 1, New molecular entity, and Type 4, New combination<br />
Type 2/3 Type 2, New active ingredient, and Type 3, New dosage form<br />
Type 2/4 Type 2, New active ingredient and Type 4, New combination<br />
Type 3/4 Type 3, New Dosage Form, and Type 4, New combination<br />
** Review Priority<br />
Priority:<br />
Review designation assigned to applications for drugs that treat serious conditions and provide significant improvements in<br />
the safety or effectiveness of the treatment, diagnosis, or prevention of serious conditions compared to available therapies<br />
Standard:<br />
Review designation assigned to applications for drugs that do not meet the priority review designation criteria<br />
<strong>The</strong> Official Publication of the Texas Society of Health-System <strong>Pharmacist</strong>s – R&E Foundation