DOWEX Ion Exchange Resins WATER CONDITIONING MANUAL
DOWEX Ion Exchange Resins WATER CONDITIONING MANUAL
DOWEX Ion Exchange Resins WATER CONDITIONING MANUAL
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Ion</strong> <strong>Exchange</strong> Resin Operational Information<br />
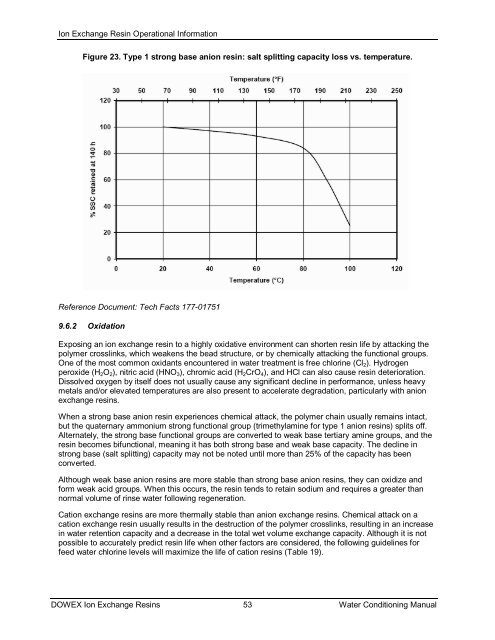
Figure 23. Type 1 strong base anion resin: salt splitting capacity loss vs. temperature.<br />
Reference Document: Tech Facts 177-01751<br />
9.6.2 Oxidation<br />
Exposing an ion exchange resin to a highly oxidative environment can shorten resin life by attacking the<br />
polymer crosslinks, which weakens the bead structure, or by chemically attacking the functional groups.<br />
One of the most common oxidants encountered in water treatment is free chlorine (Cl2). Hydrogen<br />
peroxide (H2O2), nitric acid (HNO3), chromic acid (H2CrO4), and HCl can also cause resin deterioration.<br />
Dissolved oxygen by itself does not usually cause any significant decline in performance, unless heavy<br />
metals and/or elevated temperatures are also present to accelerate degradation, particularly with anion<br />
exchange resins.<br />
When a strong base anion resin experiences chemical attack, the polymer chain usually remains intact,<br />
but the quaternary ammonium strong functional group (trimethylamine for type 1 anion resins) splits off.<br />
Alternately, the strong base functional groups are converted to weak base tertiary amine groups, and the<br />
resin becomes bifunctional, meaning it has both strong base and weak base capacity. The decline in<br />
strong base (salt splitting) capacity may not be noted until more than 25% of the capacity has been<br />
converted.<br />
Although weak base anion resins are more stable than strong base anion resins, they can oxidize and<br />
form weak acid groups. When this occurs, the resin tends to retain sodium and requires a greater than<br />
normal volume of rinse water following regeneration.<br />
Cation exchange resins are more thermally stable than anion exchange resins. Chemical attack on a<br />
cation exchange resin usually results in the destruction of the polymer crosslinks, resulting in an increase<br />
in water retention capacity and a decrease in the total wet volume exchange capacity. Although it is not<br />
possible to accurately predict resin life when other factors are considered, the following guidelines for<br />
feed water chlorine levels will maximize the life of cation resins (Table 19).<br />
<strong>DOWEX</strong> <strong>Ion</strong> <strong>Exchange</strong> <strong>Resins</strong> 53 Water Conditioning Manual