Identification and assessment of alternatives to selected phthalates
Identification and assessment of alternatives to selected phthalates
Identification and assessment of alternatives to selected phthalates
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
48<br />
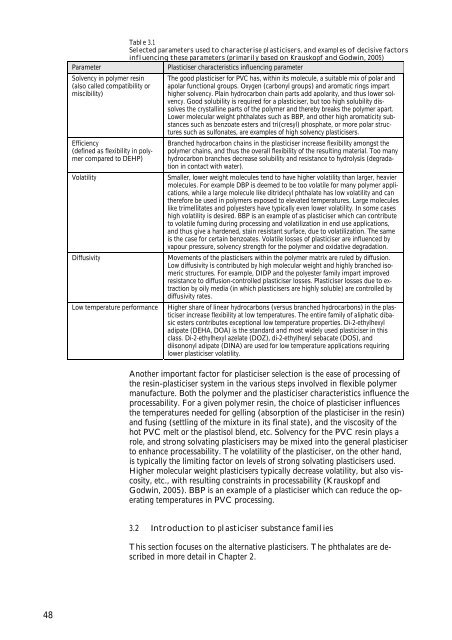
Table 3.1<br />
Selected parameters used <strong>to</strong> characterise plasticisers, <strong>and</strong> examples <strong>of</strong> decisive fac<strong>to</strong>rs<br />
influencing these parameters (primarily based on Krauskopf <strong>and</strong> Godwin, 2005)<br />
Parameter Plasticiser characteristics influencing parameter<br />
Solvency in polymer resin<br />
(also called compatibility or<br />
miscibility)<br />
Efficiency<br />
(defined as flexibility in polymer<br />
compared <strong>to</strong> DEHP)<br />
The good plasticiser for PVC has, within its molecule, a suitable mix <strong>of</strong> polar <strong>and</strong><br />
apolar functional groups. Oxygen (carbonyl groups) <strong>and</strong> aromatic rings impart<br />
higher solvency. Plain hydrocarbon chain parts add apolarity, <strong>and</strong> thus lower solvency.<br />
Good solubility is required for a plasticiser, but <strong>to</strong>o high solubility dissolves<br />
the crystalline parts <strong>of</strong> the polymer <strong>and</strong> thereby breaks the polymer apart.<br />
Lower molecular weight <strong>phthalates</strong> such as BBP, <strong>and</strong> other high aromaticity substances<br />
such as benzoate esters <strong>and</strong> tri(cresyl) phosphate, or more polar structures<br />
such as sulfonates, are examples <strong>of</strong> high solvency plasticisers.<br />
Branched hydrocarbon chains in the plasticiser increase flexibility amongst the<br />
polymer chains, <strong>and</strong> thus the overall flexibility <strong>of</strong> the resulting material. Too many<br />
hydrocarbon branches decrease solubility <strong>and</strong> resistance <strong>to</strong> hydrolysis (degradation<br />
in contact with water).<br />
Volatility Smaller, lower weight molecules tend <strong>to</strong> have higher volatility than larger, heavier<br />
molecules. For example DBP is deemed <strong>to</strong> be <strong>to</strong>o volatile for many polymer applications,<br />
while a large molecule like ditridecyl phthalate has low volatility <strong>and</strong> can<br />
therefore be used in polymers exposed <strong>to</strong> elevated temperatures. Large molecules<br />
like trimellitates <strong>and</strong> polyesters have typically even lower volatility. In some cases<br />
high volatility is desired. BBP is an example <strong>of</strong> as plasticiser which can contribute<br />
<strong>to</strong> volatile fuming during processing <strong>and</strong> volatilization in end use applications,<br />
<strong>and</strong> thus give a hardened, stain resistant surface, due <strong>to</strong> volatilization. The same<br />
is the case for certain benzoates. Volatile losses <strong>of</strong> plasticiser are influenced by<br />
vapour pressure, solvency strength for the polymer <strong>and</strong> oxidative degradation.<br />
Diffusivity Movements <strong>of</strong> the plasticisers within the polymer matrix are ruled by diffusion.<br />
Low diffusivity is contributed by high molecular weight <strong>and</strong> highly branched isomeric<br />
structures. For example, DIDP <strong>and</strong> the polyester family impart improved<br />
resistance <strong>to</strong> diffusion-controlled plasticiser losses. Plasticiser losses due <strong>to</strong> extraction<br />
by oily media (in which plasticisers are highly soluble) are controlled by<br />
diffusivity rates.<br />
Low temperature performance Higher share <strong>of</strong> linear hydrocarbons (versus branched hydrocarbons) in the plasticiser<br />
increase flexibility at low temperatures. The entire family <strong>of</strong> aliphatic dibasic<br />
esters contributes exceptional low temperature properties. Di-2-ethylhexyl<br />
adipate (DEHA, DOA) is the st<strong>and</strong>ard <strong>and</strong> most widely used plasticiser in this<br />
class. Di-2-ethylhexyl azelate (DOZ), di-2-ethylhexyl sebacate (DOS), <strong>and</strong><br />
diisononyl adipate (DINA) are used for low temperature applications requiring<br />
lower plasticiser volatility.<br />
Another important fac<strong>to</strong>r for plasticiser selection is the ease <strong>of</strong> processing <strong>of</strong><br />
the resin-plasticiser system in the various steps involved in flexible polymer<br />
manufacture. Both the polymer <strong>and</strong> the plasticiser characteristics influence the<br />
processability. For a given polymer resin, the choice <strong>of</strong> plasticiser influences<br />
the temperatures needed for gelling (absorption <strong>of</strong> the plasticiser in the resin)<br />
<strong>and</strong> fusing (settling <strong>of</strong> the mixture in its final state), <strong>and</strong> the viscosity <strong>of</strong> the<br />
hot PVC melt or the plastisol blend, etc. Solvency for the PVC resin plays a<br />
role, <strong>and</strong> strong solvating plasticisers may be mixed in<strong>to</strong> the general plasticiser<br />
<strong>to</strong> enhance processability. The volatility <strong>of</strong> the plasticiser, on the other h<strong>and</strong>,<br />
is typically the limiting fac<strong>to</strong>r on levels <strong>of</strong> strong solvating plasticisers used.<br />
Higher molecular weight plasticisers typically decrease volatility, but also viscosity,<br />
etc., with resulting constraints in processability (Krauskopf <strong>and</strong><br />
Godwin, 2005). BBP is an example <strong>of</strong> a plasticiser which can reduce the operating<br />
temperatures in PVC processing.<br />
3.2 Introduction <strong>to</strong> plasticiser substance families<br />
This section focuses on the alternative plasticisers. The <strong>phthalates</strong> are described<br />
in more detail in Chapter 2.