Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Solution Reactivity <strong>and</strong> Mechanisms through Pulse Radiolysis<br />
Sergei V. Lymar<br />
Chemistry Department, Brookhaven National Laboratory, Upton, NY 11973-5000<br />
e-mail: lymar@bnl.gov<br />
Program Scope<br />
This program applies pulse radiolysis for investigating reactive intermediates <strong>and</strong><br />
inorganic reaction mechanisms. The specific systems are selected based on their fundamental<br />
significance or importance in energy <strong>and</strong> environmental problems.<br />
The first project investigates physical chemistry of nitrogen oxides <strong>and</strong> their congeneric<br />
oxoacids <strong>and</strong> oxoanions. They play an essential role in environmental chemistry, particularly in<br />
the terrestrial nitrogen cycle, pollution, bioremediation, <strong>and</strong> ozone depletion. Nitrogen-oxygen<br />
intermediates are also central to the radiation-induced reactions that occur in nuclear fuel<br />
processing <strong>and</strong> within attendant nuclear waste. Redox <strong>and</strong> radical chemistry of the nitrate/nitrite<br />
system mediates the most of radiation-induced transformations in these environments. Equally<br />
important are the biological roles of nitrogen oxides. This research program applies timeresolved<br />
techniques for elucidation of the prospective reactions in terms of their<br />
thermodynamics, rates <strong>and</strong> mechanisms, focusing on the positive nitrogen oxidation states,<br />
whose chemistry is of the greatest current interest.<br />
The goal of the second project is to gain mechanistic insight into water oxidation<br />
catalysis through characterization of the catalyst transients involved in the catalytic cycle.<br />
Development of catalysts to carry out the four-electron water oxidation remains the greatest<br />
challenge in the solar energy utilization. Of all catalysts that have been examined, the dimeric<br />
oxo-bridged ruthenium ion ([(bpy)2(H2O)Ru-O-Ru(OH2)(bpy)2] 4+ , known as the “blue dimer”)<br />
has been the most extensively studied, but the reaction mechanisms remain to be established. The<br />
major impediment has been the difficulties in identification <strong>and</strong> characterization of the reaction<br />
intermediates. In this project, we use radiolysis techniques to generate <strong>and</strong> characterize the redox<br />
states of the catalyst involved in water oxidation by the “blue dimer” <strong>and</strong> by an all-inorganic<br />
catalyst based on cobalt hydrous oxide immobilized on silica nanoparticles that we have recently<br />
developed.<br />
Collaborators on these projects include M. Valiev (PNNL), 1 Shafirovich (NYU), 2 J. Hurst<br />
(WSU), 3 <strong>and</strong> H. Schwarz (BNL, emeritus). 4<br />
Recent Progress.<br />
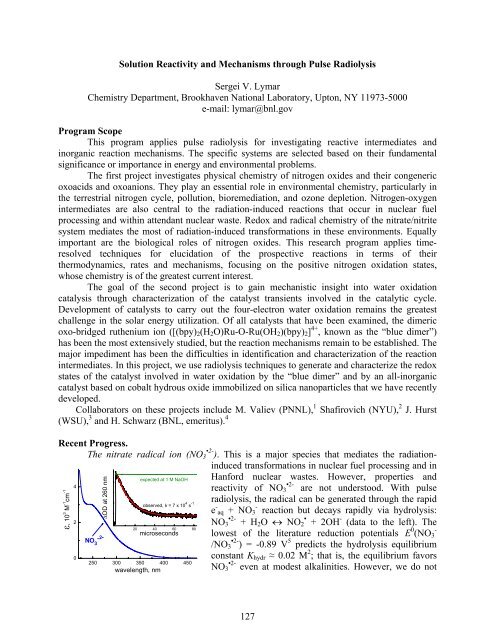
The nitrate radical ion (NO3 •2- ). This is a major species that mediates the radiationinduced<br />
transformations in nuclear fuel processing <strong>and</strong> in<br />
ε, 10 3 M -1 cm -1<br />
4<br />
2<br />
0<br />
ΔOD at 260 nm<br />
.2-<br />
NO3 expected at 1 M NaOH<br />
observed, k = 7 x 10 4 s -1<br />
20 40 60 80<br />
microseconds<br />
250 300 350 400 450<br />
wavelength, nm<br />
Hanford nuclear wastes. However, properties <strong>and</strong><br />
reactivity of NO3 •2- are not understood. With pulse<br />
radiolysis, the radical can be generated through the rapid<br />
e - aq + NO3 - reaction but decays rapidly via hydrolysis:<br />
NO3 •2- + H2O ↔ NO2 • + 2OH - (data to the left). The<br />
lowest of the literature reduction potentials E 0 (NO3 -<br />
/NO3 •2- ) = -0.89 V 5 predicts the hydrolysis equilibrium<br />
constant Khydr ≈ 0.02 M 2 ; that is, the equilibrium favors<br />
NO3 •2- even at modest alkalinities. However, we do not<br />
127