Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
entirely quenched, presumably due to reaction of the highly active Ptn with species in the air. For Pt7, air<br />
exposure leads to quenching of ORR activity, replaced by reversible redox of adventitious organic<br />
species.<br />
The instrument was modified to allow in<br />
situ electrochemical studies to be made in the<br />
lower chamber. A retractable electrochemical<br />
cell was constructed of PEEK, consisting of a 4<br />
mm diameter working section that contains a Pt<br />
counter electrode (working cell volume ≈ 15<br />
μL). One end of the working section is open,<br />
with a lip holding a 3 mm diameter O-ring that<br />
is used to seal the cell against the surface of the<br />
GCE. The other end of the working section is<br />
sealed to a reference electrode section,<br />
communicating via a fritted Vycor disk. The<br />
reference electrode was Ag/AgCl. Both<br />
working <strong>and</strong> reference sections of the cell are<br />
equipped with Teflon tubes used for injecting<br />
electrolyte, <strong>and</strong> ports to allow electrolyte<br />
solution to flow out of the cell. Electrolyte flows<br />
are controlled by a syringe pump located outside<br />
the vacuum system.<br />
To allow in situ electrochemical<br />
measurements, an electrode is first prepared by<br />
depositing Ptn on a clean, degassed GCE in<br />
UHV, which is then lowered through the triple<br />
seal into the lower chamber while the<br />
electrochemical cell is retracted. The<br />
electrochemical cell is then pushed against the<br />
surface of the GCE, sealing via the O-ring at the<br />
open end of the working section. For in situ<br />
experiments, the lower chamber was vented<br />
with high purity Ar, then the working <strong>and</strong><br />
reference sections were filled with 0.1 M<br />
HClO4, <strong>and</strong> 3 M NaCl, respectively. Cyclic<br />
voltammograms (CVs) were acquired using a<br />
CH Instruments model 600D potentiostat.<br />
The ORR results for Pt7/GCE are show<br />
in Fig. 1, in the form of CVs taken in both N2-<br />
<strong>and</strong> O2-saturated HClO4. The structure of the<br />
CV is similar to that seen both in our in situ cell<br />
<strong>and</strong> in the literature for Pt nanoparticles on<br />
GCE, <strong>and</strong> the activity per Pt atom is roughly<br />
twice that of Pt nanoparticles, probably because<br />
the small clusters have essentially all the Pt on<br />
the surface, <strong>and</strong> are highly dispersed on the<br />
GCE. Optical microscopy of the electrode after<br />
several hundred CV cycles showed no signs of<br />
any degradation.<br />
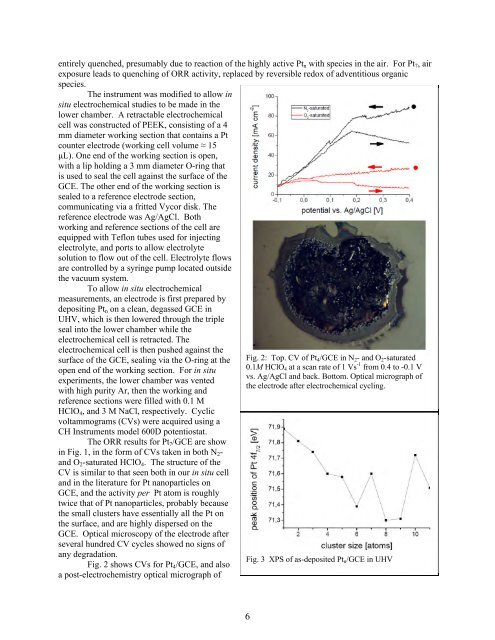
Fig. 2 shows CVs for Pt4/GCE, <strong>and</strong> also<br />
a post-electrochemistry optical micrograph of<br />
Fig. 2: Top. CV of Pt4/GCE in N2- <strong>and</strong> O2-saturated<br />
0.1M HClO4 at a scan rate of 1 Vs -1 from 0.4 to -0.1 V<br />
vs. Ag/AgCl <strong>and</strong> back. Bottom. Optical micrograph of<br />
the electrode after electrochemical cycling.<br />
Fig. 3 XPS of as-deposited Ptn/GCE in UHV<br />
6