Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
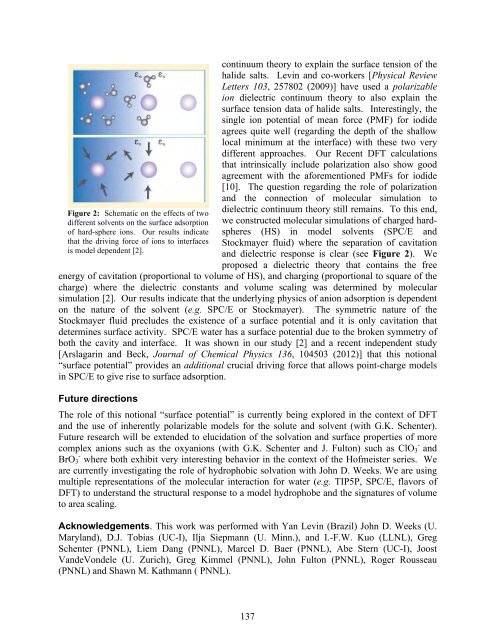
Figure 2: Schematic on the effects of two<br />
different solvents on the surface adsorption<br />
of hard-sphere ions. Our results indicate<br />
that the driving force of ions to interfaces<br />
is model dependent [2].<br />
continuum theory to explain the surface tension of the<br />
halide salts. Levin <strong>and</strong> co-workers [Physical Review<br />
Letters 103, 257802 (2009)] have used a polarizable<br />
ion dielectric continuum theory to also explain the<br />
surface tension data of halide salts. Interestingly, the<br />
single ion potential of mean force (PMF) for iodide<br />
agrees quite well (regarding the depth of the shallow<br />
local minimum at the interface) with these two very<br />
different approaches. Our Recent DFT calculations<br />
that intrinsically include polarization also show good<br />
agreement with the aforementioned PMFs for iodide<br />
[10]. The question regarding the role of polarization<br />
<strong>and</strong> the connection of molecular simulation to<br />
dielectric continuum theory still remains. To this end,<br />
we constructed molecular simulations of charged hardspheres<br />
(HS) in model solvents (SPC/E <strong>and</strong><br />
Stockmayer fluid) where the separation of cavitation<br />
<strong>and</strong> dielectric response is clear (see Figure 2). We<br />
proposed a dielectric theory that contains the free<br />
energy of cavitation (proportional to volume of HS), <strong>and</strong> charging (proportional to square of the<br />
charge) where the dielectric constants <strong>and</strong> volume scaling was determined by molecular<br />
simulation [2]. Our results indicate that the underlying physics of anion adsorption is dependent<br />
on the nature of the solvent (e.g. SPC/E or Stockmayer). The symmetric nature of the<br />
Stockmayer fluid precludes the existence of a surface potential <strong>and</strong> it is only cavitation that<br />
determines surface activity. SPC/E water has a surface potential due to the broken symmetry of<br />
both the cavity <strong>and</strong> interface. It was shown in our study [2] <strong>and</strong> a recent independent study<br />
[Arslagarin <strong>and</strong> Beck, Journal of Chemical Physics 136, 104503 (2012)] that this notional<br />
“surface potential” provides an additional crucial driving force that allows point-charge models<br />
in SPC/E to give rise to surface adsorption.<br />
Future directions<br />
The role of this notional “surface potential” is currently being explored in the context of DFT<br />
<strong>and</strong> the use of inherently polarizable models for the solute <strong>and</strong> solvent (with G.K. Schenter).<br />
Future research will be extended to elucidation of the solvation <strong>and</strong> surface properties of more<br />
complex anions such as the oxyanions (with G.K. Schenter <strong>and</strong> J. Fulton) such as ClO3 - <strong>and</strong><br />
BrO3 - where both exhibit very interesting behavior in the context of the Hofmeister series. We<br />
are currently investigating the role of hydrophobic solvation with John D. Weeks. We are using<br />
multiple representations of the molecular interaction for water (e.g. TIP5P, SPC/E, flavors of<br />
DFT) to underst<strong>and</strong> the structural response to a model hydrophobe <strong>and</strong> the signatures of volume<br />
to area scaling.<br />
Acknowledgements. This work was performed with Yan Levin (Brazil) John D. Weeks (U.<br />
Maryl<strong>and</strong>), D.J. Tobias (UC-I), Ilja Siepmann (U. Minn.), <strong>and</strong> I.-F.W. Kuo (LLNL), Greg<br />
Schenter (PNNL), Liem Dang (PNNL), Marcel D. Baer (PNNL), Abe Stern (UC-I), Joost<br />
V<strong>and</strong>eVondele (U. Zurich), Greg Kimmel (PNNL), John Fulton (PNNL), Roger Rousseau<br />
(PNNL) <strong>and</strong> Shawn M. Kathmann ( PNNL).<br />
137