Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
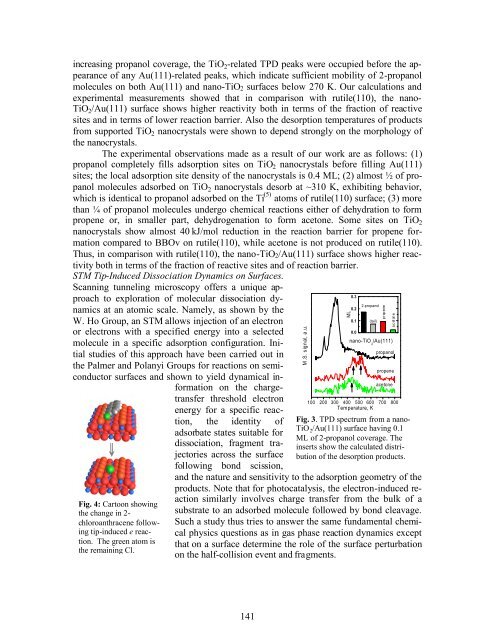
increasing propanol coverage, the TiO2-related TPD peaks were occupied before the appearance<br />
of any Au(111)-related peaks, which indicate sufficient mobility of 2-propanol<br />
molecules on both Au(111) <strong>and</strong> nano-TiO2 surfaces below 270 K. Our calculations <strong>and</strong><br />
experimental measurements showed that in comparison with rutile(110), the nano-<br />
TiO2/Au(111) surface shows higher reactivity both in terms of the fraction of reactive<br />
sites <strong>and</strong> in terms of lower reaction barrier. Also the desorption temperatures of products<br />
from supported TiO2 nanocrystals were shown to depend strongly on the morphology of<br />
the nanocrystals.<br />
The experimental observations made as a result of our work are as follows: (1)<br />
propanol completely fills adsorption sites on TiO2 nanocrystals before filling Au(111)<br />
sites; the local adsorption site density of the nanocrystals is 0.4 ML; (2) almost ½ of propanol<br />
molecules adsorbed on TiO2 nanocrystals desorb at ~310 K, exhibiting behavior,<br />
which is identical to propanol adsorbed on the Ti (5) atoms of rutile(110) surface; (3) more<br />
than ¼ of propanol molecules undergo chemical reactions either of dehydration to form<br />
propene or, in smaller part, dehydrogenation to form acetone. Some sites on TiO2<br />
nanocrystals show almost 40 kJ/mol reduction in the reaction barrier for propene formation<br />
compared to BBOv on rutile(110), while acetone is not produced on rutile(110).<br />
Thus, in comparison with rutile(110), the nano-TiO2/Au(111) surface shows higher reactivity<br />
both in terms of the fraction of reactive sites <strong>and</strong> of reaction barrier.<br />
STM Tip-Induced Dissociation Dynamics on Surfaces.<br />
Scanning tunneling microscopy offers a unique ap-<br />
proach to exploration of molecular dissociation dynamics<br />
at an atomic scale. Namely, as shown by the<br />
W. Ho Group, an STM allows injection of an electron<br />
or electrons with a specified energy into a selected<br />
molecule in a specific adsorption configuration. Initial<br />
studies of this approach have been carried out in<br />
the Palmer <strong>and</strong> Polanyi Groups for reactions on semiconductor<br />
surfaces <strong>and</strong> shown to yield dynamical information<br />
on the chargetransfer<br />
threshold electron<br />
energy for a specific reaction,<br />
the identity of<br />
adsorbate states suitable for<br />
dissociation, fragment trajectories<br />
across the surface<br />
following bond scission,<br />
Fig. 4: Cartoon showing<br />
the change in 2chloroanthracenefollowing<br />
tip-induced e reaction.<br />
The green atom is<br />
the remaining Cl.<br />
nano-TiO 2 /Au(111)<br />
propanol<br />
propene<br />
acetone<br />
100 200 300 400 500 600 700 800<br />
Temperature, K<br />
<strong>and</strong> the nature <strong>and</strong> sensitivity to the adsorption geometry of the<br />
products. Note that for photocatalysis, the electron-induced reaction<br />
similarly involves charge transfer from the bulk of a<br />
substrate to an adsorbed molecule followed by bond cleavage.<br />
Such a study thus tries to answer the same fundamental chemical<br />
physics questions as in gas phase reaction dynamics except<br />
that on a surface determine the role of the surface perturbation<br />
on the half-collision event <strong>and</strong> fragments.<br />
141<br />
M .S. s ignal, a.u.<br />
ML<br />
0.3<br />
0.2<br />
0.1<br />
0.0<br />
2-propanol<br />
(tail)<br />
Fig. 3. TPD spectrum from a nano-<br />
TiO 2/Au(111) surface having 0.1<br />
ML of 2-propanol coverage. The<br />
inserts show the calculated distribution<br />
of the desorption products.<br />
p r op e ne<br />
a c et on e