Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
Eighth Condensed Phase and Interfacial Molecular Science (CPIMS)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Future Plans<br />
In addition to<br />
the TiO2 work, we<br />
have very interesting<br />
results for Pdn on<br />
different alumina<br />
supports, <strong>and</strong> are<br />
planning to go back<br />
<strong>and</strong> look at these<br />
systems with UPS <strong>and</strong><br />
INS with the goal of<br />
underst<strong>and</strong>ing how<br />
interaction with<br />
different supports<br />
influences the valence<br />
structure in different<br />
size clusters.<br />
We also have<br />
carried out a series of<br />
non-size selected<br />
catalysis experiments<br />
(using a second, “test”<br />
instrument) focusing<br />
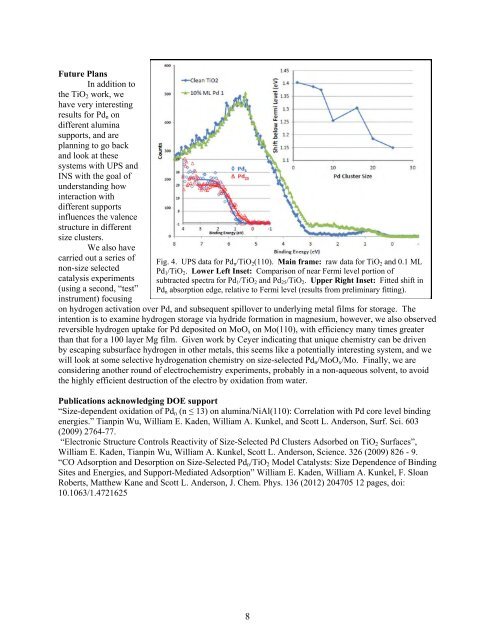
Fig. 4. UPS data for Pdn/TiO2(110). Main frame: raw data for TiO2 <strong>and</strong> 0.1 ML<br />
Pd1/TiO2. Lower Left Inset: Comparison of near Fermi level portion of<br />
subtracted spectra for Pd1/TiO2 <strong>and</strong> Pd25/TiO2. Upper Right Inset: Fitted shift in<br />
Pdn absorption edge, relative to Fermi level (results from preliminary fitting).<br />
on hydrogen activation over Pd, <strong>and</strong> subsequent spillover to underlying metal films for storage. The<br />
intention is to examine hydrogen storage via hydride formation in magnesium, however, we also observed<br />
reversible hydrogen uptake for Pd deposited on MoOx on Mo(110), with efficiency many times greater<br />
than that for a 100 layer Mg film. Given work by Ceyer indicating that unique chemistry can be driven<br />
by escaping subsurface hydrogen in other metals, this seems like a potentially interesting system, <strong>and</strong> we<br />
will look at some selective hydrogenation chemistry on size-selected Pdn/MoOx/Mo. Finally, we are<br />
considering another round of electrochemistry experiments, probably in a non-aqueous solvent, to avoid<br />
the highly efficient destruction of the electro by oxidation from water.<br />
Publications acknowledging DOE support<br />
“Size-dependent oxidation of Pdn (n ≤ 13) on alumina/NiAl(110): Correlation with Pd core level binding<br />
energies.” Tianpin Wu, William E. Kaden, William A. Kunkel, <strong>and</strong> Scott L. Anderson, Surf. Sci. 603<br />
(2009) 2764-77.<br />
“Electronic Structure Controls Reactivity of Size-Selected Pd Clusters Adsorbed on TiO2 Surfaces”,<br />
William E. Kaden, Tianpin Wu, William A. Kunkel, Scott L. Anderson, <strong>Science</strong>. 326 (2009) 826 - 9.<br />
“CO Adsorption <strong>and</strong> Desorption on Size-Selected Pdn/TiO2 Model Catalysts: Size Dependence of Binding<br />
Sites <strong>and</strong> Energies, <strong>and</strong> Support-Mediated Adsorption” William E. Kaden, William A. Kunkel, F. Sloan<br />
Roberts, Matthew Kane <strong>and</strong> Scott L. Anderson, J. Chem. Phys. 136 (2012) 204705 12 pages, doi:<br />
10.1063/1.4721625<br />
8