kinetic response of thermosetting adhesive systems to heat
kinetic response of thermosetting adhesive systems to heat
kinetic response of thermosetting adhesive systems to heat
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The slope <strong>of</strong> each regressed line reflects the <strong>adhesive</strong>s' reactivity; the steeper the slope the<br />
higher the <strong>adhesive</strong>’s reactivity. Humphrey and Ren (1989) coined the expression 'reactivity<br />
index' (Ri) <strong>to</strong> describe the bonding <strong>kinetic</strong>s <strong>of</strong> <strong>adhesive</strong> <strong>systems</strong>:<br />
Ri = -T * ln A (2)<br />
where T is the absolute Temperature (°K) and A is the rate <strong>of</strong> bond strength development<br />
(kPa*s -1 ).<br />
Linearity <strong>of</strong> the plot suggests that the bond strength development rate can be described by<br />
a first order chemical reaction. This analytical method is usually applied <strong>to</strong> chemical reactions<br />
but might be transferred <strong>to</strong> evaluate mechanical behaviour <strong>of</strong> <strong>thermosetting</strong> <strong>adhesive</strong>s. In order <strong>to</strong><br />
obtain activation energy values for the tested <strong>adhesive</strong> <strong>systems</strong> the reactivity index can be<br />
multiplied by the universal gas constant (R). Derived reactivity indices and activation energy<br />
values are presented in Table 2.<br />
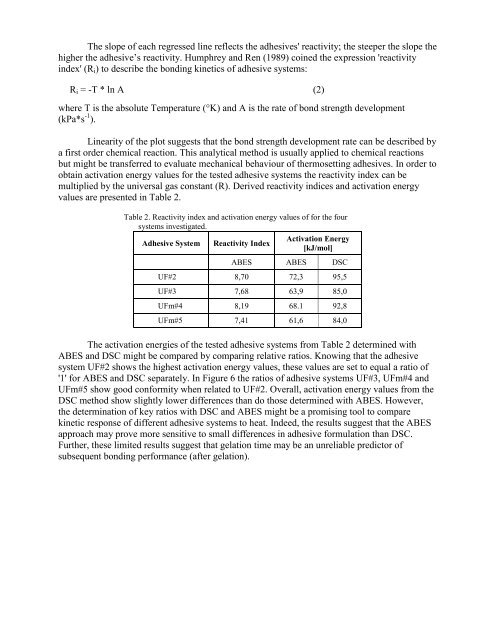
Table 2. Reactivity index and activation energy values <strong>of</strong> for the four<br />
<strong>systems</strong> investigated.<br />
Adhesive System Reactivity Index<br />
Activation Energy<br />
[kJ/mol]<br />
ABES ABES DSC<br />
UF#2 8,70 72,3 95,5<br />
UF#3 7,68 63,9 85,0<br />
UFm#4 8,19 68.1 92,8<br />
UFm#5 7,41 61,6 84,0<br />
The activation energies <strong>of</strong> the tested <strong>adhesive</strong> <strong>systems</strong> from Table 2 determined with<br />
ABES and DSC might be compared by comparing relative ratios. Knowing that the <strong>adhesive</strong><br />
system UF#2 shows the highest activation energy values, these values are set <strong>to</strong> equal a ratio <strong>of</strong><br />
'1' for ABES and DSC separately. In Figure 6 the ratios <strong>of</strong> <strong>adhesive</strong> <strong>systems</strong> UF#3, UFm#4 and<br />
UFm#5 show good conformity when related <strong>to</strong> UF#2. Overall, activation energy values from the<br />
DSC method show slightly lower differences than do those determined with ABES. However,<br />
the determination <strong>of</strong> key ratios with DSC and ABES might be a promising <strong>to</strong>ol <strong>to</strong> compare<br />
<strong>kinetic</strong> <strong>response</strong> <strong>of</strong> different <strong>adhesive</strong> <strong>systems</strong> <strong>to</strong> <strong>heat</strong>. Indeed, the results suggest that the ABES<br />
approach may prove more sensitive <strong>to</strong> small differences in <strong>adhesive</strong> formulation than DSC.<br />
Further, these limited results suggest that gelation time may be an unreliable predic<strong>to</strong>r <strong>of</strong><br />
subsequent bonding performance (after gelation).