revista perio set2010 - 3ª REV - 31-01-11.indd - Revista Sobrape
revista perio set2010 - 3ª REV - 31-01-11.indd - Revista Sobrape
revista perio set2010 - 3ª REV - 31-01-11.indd - Revista Sobrape
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
R. Periodontia - 20(3):53-59<br />
agreement with (13), for phase II trials. Twenty one subjects<br />
(10 males and 11 female) completed the <strong>perio</strong>d of study. Two<br />
individuals were excluded from the search before the second<br />
exam. One subject visited other dentist during the study<br />
and the other individual had allergy to the product tested.<br />
One subject was excluded after the second exam, because<br />
of taking antibiotics due to illness. One person gave up the<br />
study due to personal issues. Data related to behavior and<br />
habits of life of each patient were recorded (Table 1).<br />
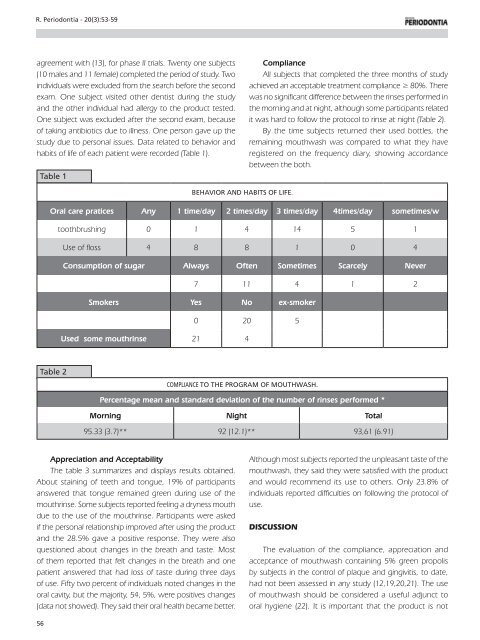
Table 1<br />
Compliance<br />
All subjects that completed the three months of study<br />
achieved an acceptable treatment compliance ≥ 80%. There<br />
was no significant difference between the rinses performed in<br />
the morning and at night, although some participants related<br />
it was hard to follow the protocol to rinse at night (Table 2).<br />
By the time subjects returned their used bottles, the<br />
remaining mouthwash was compared to what they have<br />
registered on the frequency diary, showing accordance<br />
between the both.<br />
Behavior and habits of life.<br />
Oral care pratices Any 1 time/day 2 times/day 3 times/day 4times/day sometimes/w<br />
toothbrushing 0 1 4 14 5 1<br />
Use of floss 4 8 8 1 0 4<br />
Consumption of sugar Always Often Sometimes Scarcely Never<br />
7 11 4 1 2<br />
Smokers Yes No ex-smoker<br />
0 20 5<br />
Used some mouthrinse 21 4<br />
Table 2<br />
Compliance to the program of mouthwash.<br />
Percentage mean and standard deviation of the number of rinses performed *<br />
Morning Night Total<br />
95.33 (3.7)** 92 (12.1)** 93,61 (6.91)<br />
Appreciation and Acceptability<br />
The table 3 summarizes and displays results obtained.<br />
About staining of teeth and tongue, 19% of participants<br />
answered that tongue remained green during use of the<br />
mouthrinse. Some subjects reported feeling a dryness mouth<br />
due to the use of the mouthrinse. Participants were asked<br />
if the personal relationship improved after using the product<br />
and the 28.5% gave a positive response. They were also<br />
questioned about changes in the breath and taste. Most<br />
of them reported that felt changes in the breath and one<br />
patient answered that had loss of taste during three days<br />
of use. Fifty two percent of individuals noted changes in the<br />
oral cavity, but the majority, 54, 5%, were positives changes<br />
(data not showed). They said their oral health became better.<br />
Although most subjects reported the unpleasant taste of the<br />
mouthwash, they said they were satisfied with the product<br />
and would recommend its use to others. Only 23.8% of<br />
individuals reported difficulties on following the protocol of<br />
use.<br />
DISCUSSION<br />
The evaluation of the compliance, appreciation and<br />
acceptance of mouthwash containing 5% green propolis<br />
by subjects in the control of plaque and gingivitis, to date,<br />
had not been assessed in any study (12,19,20,21). The use<br />
of mouthwash should be considered a useful adjunct to<br />
oral hygiene (22). It is important that the product is not<br />
56<br />
<strong>revista</strong> <strong>perio</strong> <strong>set2<strong>01</strong>0</strong> - 3ª <strong>REV</strong> - <strong>31</strong>-<strong>01</strong>-<strong>11.indd</strong> 56 <strong>31</strong>/1/2<strong>01</strong>1 23:35:29