Sanitetsst tte til forsvarets internationale missioner
Sanitetsst tte til forsvarets internationale missioner
Sanitetsst tte til forsvarets internationale missioner
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
UKLASSIFICERET<br />
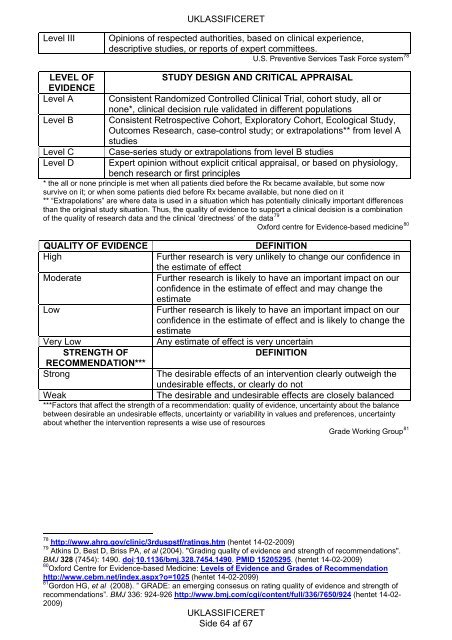
Level III Opinions of respected authorities, based on clinical experience,<br />
descriptive studies, or reports of expert commi<strong>tte</strong>es.<br />
U.S. Preventive Services Task Force system 78<br />
LEVEL OF<br />
STUDY DESIGN AND CRITICAL APPRAISAL<br />
EVIDENCE<br />
Level A Consistent Randomized Controlled Clinical Trial, cohort study, all or<br />
none*, clinical decision rule validated in different populations<br />
Level B Consistent Retrospective Cohort, Exploratory Cohort, Ecological Study,<br />
Outcomes Research, case-control study; or extrapolations** from level A<br />
studies<br />
Level C Case-series study or extrapolations from level B studies<br />
Level D Expert opinion without explicit critical appraisal, or based on physiology,<br />
bench research or first principles<br />
* the all or none principle is met when all patients died before the Rx became available, but some now<br />
survive on it; or when some patients died before Rx became available, but none died on it<br />
** “Extrapolations” are where data is used in a situation which has potentially clinically important differences<br />
than the original study situation. Thus, the quality of evidence to support a clinical decision is a combination<br />
of the quality of research data and the clinical ‘directness’ of the data 79<br />
Oxford centre for Evidence-based medicine 80<br />
QUALITY OF EVIDENCE DEFINITION<br />
High Further research is very unlikely to change our confidence in<br />
the estimate of effect<br />
Moderate Further research is likely to have an important impact on our<br />
confidence in the estimate of effect and may change the<br />
estimate<br />
Low Further research is likely to have an important impact on our<br />
confidence in the estimate of effect and is likely to change the<br />
estimate<br />
Very Low Any estimate of effect is very uncertain<br />
STRENGTH OF<br />
DEFINITION<br />
RECOMMENDATION***<br />
Strong The desirable effects of an intervention clearly outweigh the<br />
undesirable effects, or clearly do not<br />
Weak The desirable and undesirable effects are closely balanced<br />
***Factors that affect the strength of a recommendation: quality of evidence, uncertainty about the balance<br />
between desirable an undesirable effects, uncertainty or variability in values and preferences, uncertainty<br />
about whether the intervention represents a wise use of resources<br />
Grade Working Group 81<br />
78 http://www.ahrq.gov/clinic/3rduspstf/ratings.htm (hentet 14-02-2009)<br />
79 Atkins D, Best D, Briss PA, et al (2004). "Grading quality of evidence and strength of recommendations".<br />
BMJ 328 (7454): 1490. doi:10.1136/bmj.328.7454.1490. PMID 15205295. (hentet 14-02-2009)<br />
80 Oxford Centre for Evidence-based Medicine: Levels of Evidence and Grades of Recommendation<br />
http://www.cebm.net/index.aspx?o=1025 (hentet 14-02-2099)<br />
81 Gordon HG, et al (2008). ” GRADE: an emerging consesus on rating quality of evidence and strength of<br />
recommendations”. BMJ 336: 924-926 http://www.bmj.com/cgi/content/full/336/7650/924 (hentet 14-02-<br />
2009)<br />
UKLASSIFICERET<br />
Side 64 af 67