antimony - Sciencemadness.org
antimony - Sciencemadness.org
antimony - Sciencemadness.org
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
88<br />
THE METALLURGY OF ANTIMONY.<br />
is introduced into a blast furnace or a cupola with 10 per cent, of<br />
coal. If the mineral is an oxide, then 25 per cent, of coal is required.<br />
In immediate connection with the furnace or cupola is a chamber<br />
for the condensation of the volatile oxide thus produced, and beyond<br />
it a reservoir containing water for the condensation of the last trace<br />
of the volatile trioxide. The practical working of this process may<br />
thus be summarised:—200 lbs. of coal are burned in the furnace or<br />
cupola; on it is put one ton of sulphide ore or 800 lbs. of oxide ore;<br />
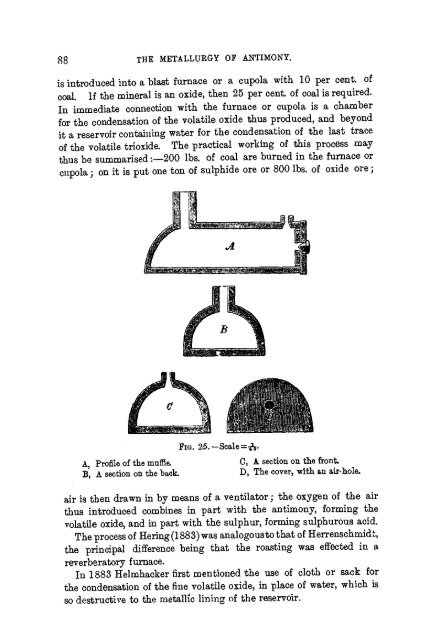
FIG. 25.—Scale=^.<br />
A, Profile of the muffle. C, A section on the front<br />
B*, A section on the back. D, The cover, with an air-hole.<br />
air is then drawn in by means of a ventilator; the oxygen of the air<br />
thus introduced combines in part with the <strong>antimony</strong>, forming the<br />
volatile oxide, and in part with the sulphur, forming sulphurous acid.<br />
The process of Hering(1883) was analogous to that of Herrenschmidt,<br />
the principal difference being that the roasting was effected in a<br />
reverberatory furnace.<br />
In 1883 Helmhacker first mentioned the use of cloth or sack for<br />
the condensation of the fine volatile oxide, in place of water, which is<br />
so destructive to the metallic lining of the reservoir.