Procedure for assessing the acceptability, in principle, of vaccines ...
Procedure for assessing the acceptability, in principle, of vaccines ...
Procedure for assessing the acceptability, in principle, of vaccines ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
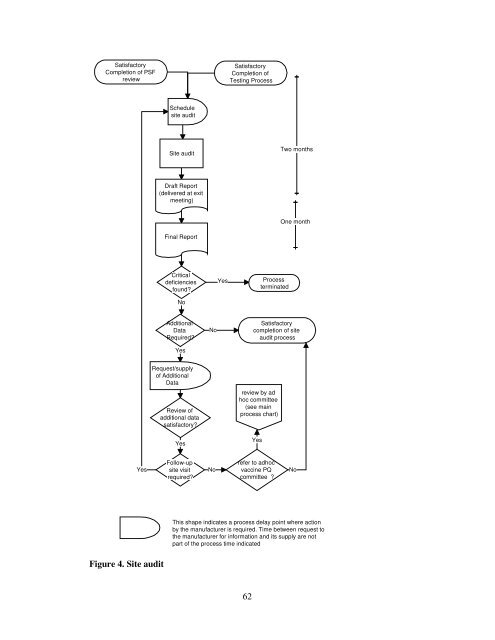
Satisfactory<br />
Completion <strong>of</strong> PSF<br />
review<br />
Yes<br />
Figure 4. Site audit<br />
Schedule<br />
site audit<br />
Site audit<br />
Draft Report<br />
(delivered at exit<br />
meet<strong>in</strong>g)<br />
F<strong>in</strong>al Report<br />
Critical<br />
deficiencies<br />
found?<br />
No<br />
Additional<br />
Data<br />
Required?<br />
Yes<br />
Request/supply<br />
<strong>of</strong> Additional<br />
Data<br />
Review <strong>of</strong><br />
additional data<br />
satisfactory?<br />
Yes<br />
Follow-up<br />
site visit<br />
required?<br />
No<br />
No<br />
Yes<br />
Satisfactory<br />
Completion <strong>of</strong><br />
Test<strong>in</strong>g Process<br />
62<br />
Process<br />
term<strong>in</strong>ated<br />
Satisfactory<br />
completion <strong>of</strong> site<br />
audit process<br />
review by ad<br />
hoc committee<br />
(see ma<strong>in</strong><br />
process chart)<br />
Yes<br />
refer to adhoc<br />
vacc<strong>in</strong>e PQ<br />
committee ?<br />
Two months<br />
One month<br />
This shape <strong>in</strong>dicates a process delay po<strong>in</strong>t where action<br />
by <strong>the</strong> manufacturer is required. Time between request to<br />
<strong>the</strong> manufacturer <strong>for</strong> <strong>in</strong><strong>for</strong>mation and its supply are not<br />
part <strong>of</strong> <strong>the</strong> process time <strong>in</strong>dicated<br />
No