Procedure for assessing the acceptability, in principle, of vaccines ...
Procedure for assessing the acceptability, in principle, of vaccines ...
Procedure for assessing the acceptability, in principle, of vaccines ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1<br />
2<br />
3<br />
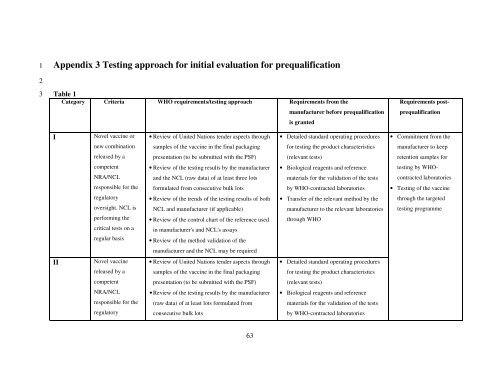
Appendix 3 Test<strong>in</strong>g approach <strong>for</strong> <strong>in</strong>itial evaluation <strong>for</strong> prequalification<br />
Table 1<br />
Category Criteria WHO requirements/test<strong>in</strong>g approach Requirements from <strong>the</strong><br />
I<br />
II<br />
Novel vacc<strong>in</strong>e or<br />
new comb<strong>in</strong>ation<br />
released by a<br />
competent<br />
NRA/NCL<br />
responsible <strong>for</strong> <strong>the</strong><br />
regulatory<br />
oversight. NCL is<br />
per<strong>for</strong>m<strong>in</strong>g <strong>the</strong><br />
critical tests on a<br />
regular basis<br />
Novel vacc<strong>in</strong>e<br />
released by a<br />
competent<br />
NRA/NCL<br />
responsible <strong>for</strong> <strong>the</strong><br />
regulatory<br />
• Review <strong>of</strong> United Nations tender aspects through<br />
samples <strong>of</strong> <strong>the</strong> vacc<strong>in</strong>e <strong>in</strong> <strong>the</strong> f<strong>in</strong>al packag<strong>in</strong>g<br />
presentation (to be submitted with <strong>the</strong> PSF)<br />
• Review <strong>of</strong> <strong>the</strong> test<strong>in</strong>g results by <strong>the</strong> manufacturer<br />
and <strong>the</strong> NCL (raw data) <strong>of</strong> at least three lots<br />
<strong>for</strong>mulated from consecutive bulk lots<br />
• Review <strong>of</strong> <strong>the</strong> trends <strong>of</strong> <strong>the</strong> test<strong>in</strong>g results <strong>of</strong> both<br />
NCL and manufacturer (if applicable)<br />
• Review <strong>of</strong> <strong>the</strong> control chart <strong>of</strong> <strong>the</strong> reference used<br />
<strong>in</strong> manufacturer's and NCL's assays<br />
• Review <strong>of</strong> <strong>the</strong> method validation <strong>of</strong> <strong>the</strong><br />
manufacturer and <strong>the</strong> NCL may be required<br />
• Review <strong>of</strong> United Nations tender aspects through<br />
samples <strong>of</strong> <strong>the</strong> vacc<strong>in</strong>e <strong>in</strong> <strong>the</strong> f<strong>in</strong>al packag<strong>in</strong>g<br />
presentation (to be submitted with <strong>the</strong> PSF)<br />
• Review <strong>of</strong> <strong>the</strong> test<strong>in</strong>g results by <strong>the</strong> manufacturer<br />
(raw data) <strong>of</strong> at least lots <strong>for</strong>mulated from<br />
consecutive bulk lots<br />
63<br />
manufacturer be<strong>for</strong>e prequalification<br />
is granted<br />
• Detailed standard operat<strong>in</strong>g procedures<br />
<strong>for</strong> test<strong>in</strong>g <strong>the</strong> product characteristics<br />
(relevant tests)<br />
• Biological reagents and reference<br />
materials <strong>for</strong> <strong>the</strong> validation <strong>of</strong> <strong>the</strong> tests<br />
by WHO-contracted laboratories<br />
• Transfer <strong>of</strong> <strong>the</strong> relevant method by <strong>the</strong><br />
manufacturer to <strong>the</strong> relevant laboratories<br />
through WHO<br />
• Detailed standard operat<strong>in</strong>g procedures<br />
<strong>for</strong> test<strong>in</strong>g <strong>the</strong> product characteristics<br />
(relevant tests)<br />
• Biological reagents and reference<br />
materials <strong>for</strong> <strong>the</strong> validation <strong>of</strong> <strong>the</strong> tests<br />
by WHO-contracted laboratories<br />
Requirements post-<br />
prequalification<br />
• Commitment from <strong>the</strong><br />
manufacturer to keep<br />
retention samples <strong>for</strong><br />
test<strong>in</strong>g by WHO-<br />
contracted laboratories<br />
• Test<strong>in</strong>g <strong>of</strong> <strong>the</strong> vacc<strong>in</strong>e<br />
through <strong>the</strong> targeted<br />
test<strong>in</strong>g programme