- Page 4:
GENE CLONING AND DNA ANALYSIS
- Page 10:
This edition first published 2010,
- Page 16:
Contents CONTENTS Preface to the Si
- Page 20:
Contents ix 4.3 Ligation—joining
- Page 24:
Contents xi 8 How to Obtain a Clone
- Page 28:
Contents xiii 11.3 Identifying and
- Page 32:
Contents xv 15.1.2 Herbicide resist
- Page 36:
PART I The Basic Principles of Gene
- Page 42:
4 Part I The Basic Principles of Ge
- Page 46:
6 1.4 What is PCR? Figure 1.2 The b
- Page 50:
8 Figure 1.3 Cloning allows individ
- Page 54:
10 Figure 1.5 Gene isolation by PCR
- Page 58:
12 Further reading FURTHER READING
- Page 62:
14 Figure 2.1 Plasmids: independent
- Page 66:
16 Figure 2.4 Plasmid transfer by c
- Page 70:
18 Figure 2.6 Capsid components Pha
- Page 74:
20 Figure 2.7 λ phage particle att
- Page 78:
22 (a) The linear form of the λ DN
- Page 82:
24 Part I The Basic Principles of G
- Page 86:
26 Bacterial culture Table 3.1 Cent
- Page 90:
28 Bacterial culture Figure 3.3 Har
- Page 94:
30 Figure 3.6 Removal of protein co
- Page 98:
32 Figure 3.8 Collecting DNA by eth
- Page 102:
34 Figure 3.10 DNA purification by
- Page 106:
36 Figure 3.12 Two conformations of
- Page 110:
38 Figure 3.15 Partial unwinding of
- Page 114:
40 Figure 3.18 Preparation of a pha
- Page 118:
42 Figure 3.21 Collection of phage
- Page 122:
44 Further reading FURTHER READING
- Page 126:
46 Part I The Basic Principles of G
- Page 130:
48 Figure 4.3 The reactions catalyz
- Page 134:
50 Figure 4.6 (a) Alkaline phosphat
- Page 138:
52 Figure 4.8 (a) Restriction of ph
- Page 142:
54 Figure 4.9 (a) Production of blu
- Page 146:
56 Table 4.2 A 10 × buffer suitabl
- Page 150:
58 Figure 4.13 Visualizing DNA band
- Page 154:
60 Figure 4.15 Using a restriction
- Page 158:
62 Figure 4.17 The influence of DNA
- Page 162:
64 Figure 4.20 (a) Ligating blunt e
- Page 166:
66 Figure 4.23 Adaptors and the pot
- Page 170:
68 Figure 4.25 The use of adaptors:
- Page 174:
70 Figure 4.28 (a) The ends of the
- Page 178:
72 Chapter 5 Introduction of DNA in
- Page 182:
74 Part I The Basic Principles of G
- Page 186:
76 Figure 5.4 Normal E. coli cell (
- Page 190:
78 Figure 5.8 (b) Replica plating W
- Page 194:
80 Figure 5.10 (a) The role of the
- Page 198:
82 Figure 5.11 (a) A single-strain
- Page 202:
84 Figure 5.13 Strategies for the s
- Page 206:
86 Figure 5.14 Figure 5.15 (a) Prec
- Page 210:
88 Chapter 6 Cloning Vectors for E.
- Page 214:
90 during purification. pBR322 is 4
- Page 218:
92 (a) pUC8 (b) Restriction sites i
- Page 222:
94 Figure 6.5 The M13 genome, showi
- Page 226:
96 not totally disrupt the lacZ′
- Page 230:
98 Figure 6.10 The λ genetic map,
- Page 234:
100 (a) Cloning with a λ replaceme
- Page 238:
102 Figure 6.15 (a) A typical cosmi
- Page 242:
104 capsid structure. Cosmid-type v
- Page 246:
106 Figure 7.1 The yeast 2 µm plas
- Page 250:
108 Figure 7.4 Cloning with an E. c
- Page 254:
110 Figure 7.7 Chromosome structure
- Page 258:
112 for instance to examine the seg
- Page 262:
114 Figure 7.10 The Ti plasmid and
- Page 266:
116 (a) Wound infection by recombin
- Page 270:
118 Figure 7.15 Direct gene transfe
- Page 274:
120 Caulimovirus vectors Although o
- Page 278:
122 Figure 7.18 Cloning in Drosophi
- Page 282:
124 cause the death of the mouse ce
- Page 286: 126 Chapter 8 How to Obtain a Clone
- Page 290: 128 Figure 8.2 The basic strategies
- Page 294: 130 Figure 8.4 (a) Construction of
- Page 298: 132 Figure 8.5 Preparation of a gen
- Page 302: 134 Figure 8.7 One possible scheme
- Page 306: 136 Figure 8.10 The structure of α
- Page 310: 138 Figure 8.12 Two methods for the
- Page 314: 140 Figure 8.15 A simplified scheme
- Page 318: 142 Figure 8.17 Heterologous probin
- Page 322: 144 skills (Figure 8.19b). The same
- Page 326: 146 The problem of gene expression
- Page 330: 148 Figure 9.1 Hybridization of the
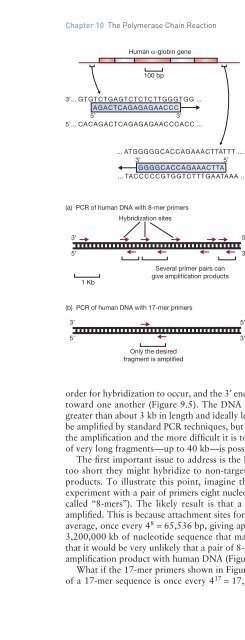
- Page 334: 150 Figure 9.3 Figure 9.4 5’ 3’
- Page 340: Chapter 10 The Polymerase Chain Rea
- Page 344: Chapter 10 The Polymerase Chain Rea
- Page 348: Chapter 10 The Polymerase Chain Rea
- Page 352: Chapter 10 The Polymerase Chain Rea
- Page 356: Chapter 10 The Polymerase Chain Rea
- Page 364: Chapter 10 Sequencing Genes and Gen
- Page 368: Chapter 10 Sequencing Genes and Gen
- Page 372: Chapter 10 Sequencing Genes and Gen
- Page 376: Chapter 10 Sequencing Genes and Gen
- Page 380: Chapter 10 Sequencing Genes and Gen
- Page 384: Chapter 10 Sequencing Genes and Gen
- Page 388:
Chapter 10 Sequencing Genes and Gen
- Page 392:
Chapter 10 Sequencing Genes and Gen
- Page 396:
Chapter 10 Sequencing Genes and Gen
- Page 400:
Chapter 10 Sequencing Genes and Gen
- Page 404:
Chapter 11 Studying Gene Expression
- Page 408:
Chapter 11 Studying Gene Expression
- Page 412:
Chapter 11 Studying Gene Expression
- Page 416:
Chapter 11 Studying Gene Expression
- Page 420:
Chapter 11 Studying Gene Expression
- Page 424:
Chapter 11 Studying Gene Expression
- Page 428:
Chapter 11 Studying Gene Expression
- Page 432:
Chapter 11 Studying Gene Expression
- Page 436:
Chapter 11 Studying Gene Expression
- Page 440:
Chapter 11 Studying Gene Expression
- Page 444:
Chapter 11 Studying Gene Expression
- Page 448:
Chapter 12 Studying Genomes Chapter
- Page 452:
Chapter 12 Studying Genomes 209 Fig
- Page 456:
Chapter 12 Studying Genomes 211 Fig
- Page 460:
Chapter 12 Studying Genomes 213 (a)
- Page 464:
Chapter 12 Studying Genomes 215 12.
- Page 468:
Chapter 12 Studying Genomes 217 C G
- Page 472:
Chapter 12 Studying Genomes 219 Fig
- Page 476:
Chapter 12 Studying Genomes 221 Fig
- Page 480:
PART III The Applications of Gene C
- Page 486:
226 Figure 13.1 Two different syste
- Page 490:
228 Figure 13.3 The three most impo
- Page 494:
230 Figure 13.6 Strong and weak pro
- Page 498:
232 Part III The Applications of Ge
- Page 502:
234 Figure 13.12 Fusion protein bou
- Page 506:
236 organism has a bias toward pref
- Page 510:
238 Figure 13.16 Part III The Appli
- Page 514:
240 Figure 13.18 Crystalline inclus
- Page 518:
242 Figure 13.20 Recombinant protei
- Page 522:
244 s Part III The Applications of
- Page 526:
246 14.1.1 Recombinant insulin Figu
- Page 530:
248 Figure 14.2 The synthesis of re
- Page 534:
250 Figure 14.5 (a) The factor VIII
- Page 538:
252 Figure 14.7 Part III The Applic
- Page 542:
254 Figure 14.8 Part III The Applic
- Page 546:
256 Part III The Applications of Ge
- Page 550:
258 Figure 14.10 Part III The Appli
- Page 554:
260 Figure 14.11 Differentiation of
- Page 558:
262 Part III The Applications of Ge
- Page 562:
264 Chapter 15 Gene Cloning and DNA
- Page 566:
266 Table 15.1 Part III The Applica
- Page 570:
268 Figure 15.3 Positional effects.
- Page 574:
270 (a) Production of two toxins (c
- Page 578:
272 (a) Detoxification of glyphosat
- Page 582:
274 are being produced by transferr
- Page 586:
276 Figure 15.10 The differences in
- Page 590:
278 Figure 15.11 DNA excision by th
- Page 594:
280 Part III The Applications of Ge
- Page 598:
282 Chapter 16 Gene Cloning and DNA
- Page 602:
284 Figure 16.1 Genetic fingerprint
- Page 606:
286 Figure 16.3 Inheritance of STR
- Page 610:
288 Figure 16.5 Part III The Applic
- Page 614:
290 Figure 16.6 Sex identification
- Page 618:
292 Figure 16.8 Part III The Applic
- Page 622:
294 Part III The Applications of Ge
- Page 626:
296 Figure 16.11 Origin of agricult
- Page 630:
298 Glossary GLOSSARY 2 Fm circle A
- Page 634:
300 Glossary Chromosome walking A t
- Page 638:
302 Glossary Ethanol precipitation
- Page 642:
304 Glossary In vitro mutagenesis A
- Page 646:
306 Glossary Nick translation The r
- Page 650:
308 Glossary Proteome The entire pr
- Page 654:
310 Glossary Single nucleotide poly
- Page 658:
312 INDEX Index (g = glossary, f =
- Page 662:
314 cloning vectors (continued) exp
- Page 666:
316 herpes simplex virus glycoprote
- Page 670:
318 polyethylene glycol, see PEG po
- Page 674:
320 T m , 153, 305g tobacco, 269 TO