use of metal templates for microcavity formation in alumina
use of metal templates for microcavity formation in alumina
use of metal templates for microcavity formation in alumina
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Densification Rate (1/ o C)<br />
0,009<br />
0,007<br />
0,005<br />
0,003<br />
0,001<br />
800 900 1000 1100 1200 1300 1400 1500 1600<br />
-0,001<br />
-0,003<br />
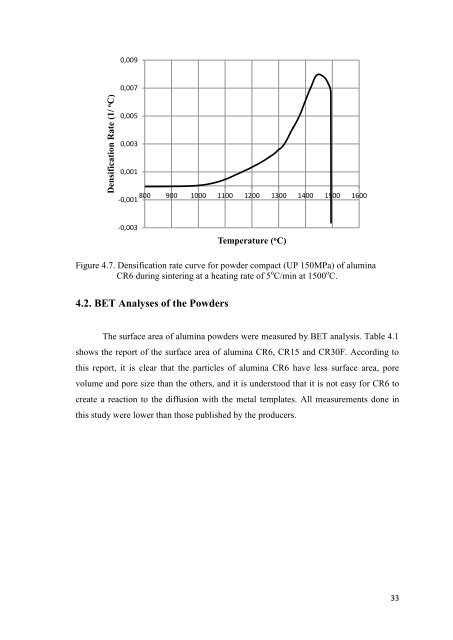
Figure 4.7. Densification rate curve <strong>for</strong> powder compact (UP 150MPa) <strong>of</strong> alum<strong>in</strong>a<br />
CR6 dur<strong>in</strong>g s<strong>in</strong>ter<strong>in</strong>g at a heat<strong>in</strong>g rate <strong>of</strong> 5 o C/m<strong>in</strong> at 1500 o C.<br />
4.2. BET Analyses <strong>of</strong> the Powders<br />
Temperature ( o C)<br />
The surface area <strong>of</strong> alum<strong>in</strong>a powders were measured by BET analysis. Table 4.1<br />
shows the report <strong>of</strong> the surface area <strong>of</strong> alum<strong>in</strong>a CR6, CR15 and CR30F. Accord<strong>in</strong>g to<br />
this report, it is clear that the particles <strong>of</strong> alum<strong>in</strong>a CR6 have less surface area, pore<br />
volume and pore size than the others, and it is understood that it is not easy <strong>for</strong> CR6 to<br />
create a reaction to the diffusion with the <strong>metal</strong> <strong>templates</strong>. All measurements done <strong>in</strong><br />
this study were lower than those published by the producers.<br />
33