use of metal templates for microcavity formation in alumina
use of metal templates for microcavity formation in alumina
use of metal templates for microcavity formation in alumina
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Al2O3<br />
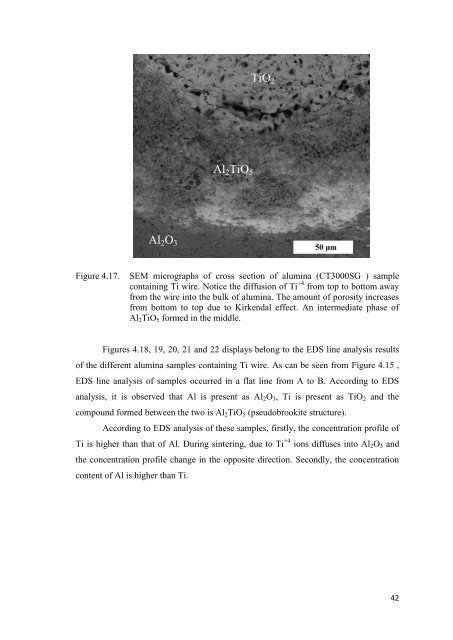
Figure 4.17. SEM micrographs <strong>of</strong> cross section <strong>of</strong> alum<strong>in</strong>a (CT3000SG ) sample<br />
conta<strong>in</strong><strong>in</strong>g Ti wire. Notice the diffusion <strong>of</strong> Ti +4 from top to bottom away<br />
from the wire <strong>in</strong>to the bulk <strong>of</strong> alum<strong>in</strong>a. The amount <strong>of</strong> porosity <strong>in</strong>creases<br />
from bottom to top due to Kirkendal effect. An <strong>in</strong>termediate phase <strong>of</strong><br />
Al2TiO5 <strong>for</strong>med <strong>in</strong> the middle.<br />
Figures 4.18, 19, 20, 21 and 22 displays belong to the EDS l<strong>in</strong>e analysis results<br />
<strong>of</strong> the different alum<strong>in</strong>a samples conta<strong>in</strong><strong>in</strong>g Ti wire. As can be seen from Figure 4.15 ,<br />
EDS l<strong>in</strong>e analysis <strong>of</strong> samples occurred <strong>in</strong> a flat l<strong>in</strong>e from A to B. Accord<strong>in</strong>g to EDS<br />
analysis, it is observed that Al is present as Al2O3, Ti is present as TiO2 and the<br />
compound <strong>for</strong>med between the two is Al2TiO5 (pseudobrookite structure).<br />
Accord<strong>in</strong>g to EDS analysis <strong>of</strong> these samples, firstly, the concentration pr<strong>of</strong>ile <strong>of</strong><br />
Ti is higher than that <strong>of</strong> Al. Dur<strong>in</strong>g s<strong>in</strong>ter<strong>in</strong>g, due to Ti +4 ions diff<strong>use</strong>s <strong>in</strong>to Al2O3 and<br />
the concentration pr<strong>of</strong>ile change <strong>in</strong> the opposite direction. Secondly, the concentration<br />
content <strong>of</strong> Al is higher than Ti.<br />
Al2TiO5<br />
TiO2<br />
42