Rudolph MG
Rudolph MG
Rudolph MG
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Annu. Rev. Immunol. 2006.24:419-466. Downloaded from arjournals.annualreviews.org<br />
by CAMBRIDGE UNIVERSITY on 04/04/10. For personal use only.<br />
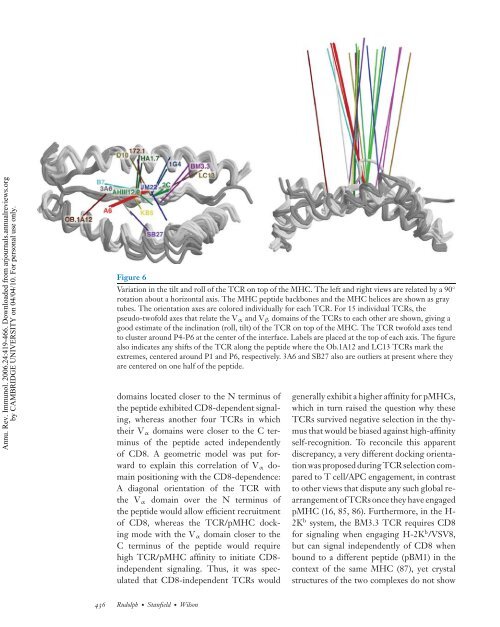
Figure 6<br />
Variation in the tilt and roll of the TCR on top of the MHC. The left and right views are related by a 90 ◦<br />
rotation about a horizontal axis. The MHC peptide backbones and the MHC helices are shown as gray<br />
tubes. The orientation axes are colored individually for each TCR. For 15 individual TCRs, the<br />
pseudo-twofold axes that relate the Vα and Vβ domains of the TCRs to each other are shown, giving a<br />
good estimate of the inclination (roll, tilt) of the TCR on top of the MHC. The TCR twofold axes tend<br />
to cluster around P4-P6 at the center of the interface. Labels are placed at the top of each axis. The figure<br />
also indicates any shifts of the TCR along the peptide where the Ob.1A12 and LC13 TCRs mark the<br />
extremes, centered around P1 and P6, respectively. 3A6 and SB27 also are outliers at present where they<br />
are centered on one half of the peptide.<br />
domains located closer to the N terminus of<br />
the peptide exhibited CD8-dependent signaling,<br />
whereas another four TCRs in which<br />
their Vα domains were closer to the C terminus<br />
of the peptide acted independently<br />
of CD8. A geometric model was put forward<br />
to explain this correlation of Vα domain<br />
positioning with the CD8-dependence:<br />
A diagonal orientation of the TCR with<br />
the Vα domain over the N terminus of<br />
the peptide would allow efficient recruitment<br />
of CD8, whereas the TCR/pMHC docking<br />
mode with the Vα domain closer to the<br />
C terminus of the peptide would require<br />
high TCR/pMHC affinity to initiate CD8independent<br />
signaling. Thus, it was speculated<br />
that CD8-independent TCRs would<br />
436 <strong>Rudolph</strong>· Stanfield· Wilson<br />
generally exhibit a higher affinity for pMHCs,<br />
which in turn raised the question why these<br />
TCRs survived negative selection in the thymus<br />
that would be biased against high-affinity<br />
self-recognition. To reconcile this apparent<br />
discrepancy, a very different docking orientation<br />
was proposed during TCR selection compared<br />
to T cell/APC engagement, in contrast<br />
to other views that dispute any such global rearrangement<br />
of TCRs once they have engaged<br />
pMHC (16, 85, 86). Furthermore, in the H-<br />
2K b system, the BM3.3 TCR requires CD8<br />
for signaling when engaging H-2K b /VSV8,<br />
but can signal independently of CD8 when<br />
bound to a different peptide (pBM1) in the<br />
context of the same MHC (87), yet crystal<br />
structures of the two complexes do not show