- Page 1 and 2:
CHAPTER I PROPERTIES OF EXPLOSIVES
- Page 3 and 4:
HIGH EXPLOSIVES 3 they are heated o
- Page 5 and 6:
HIGH EXPLOSIVES 5 black match may b
- Page 7 and 8:
A COMPLETE ROUND OF AMMUNITION 7 f
- Page 9 and 10:
A COMPLETE ROUND OF AMMUNITION 9 11
- Page 11 and 12:
DETONATING FUSE 11 ting upon, say,
- Page 13 and 14:
DETONATING FUSE 13 branch line squa
- Page 15 and 16:
FIGURE 8. Pierre-Eugene Marcellin B
- Page 17 and 18:
VELOCITY OF DETONATION 17 placed up
- Page 19 and 20:
FIGURE 10 Charles Edward Munroe (18
- Page 21 and 22:
Sensitivity Tests SENSITIVITY TESTS
- Page 23 and 24:
TESTS OF EXPLOSIVE POWER AND BRISAN
- Page 25 and 26:
TESTS OF EXPLOSIVE POWER AND BRISAN
- Page 27 and 28:
TESTS OF EXPLOSIVE POWER AND BRISAN
- Page 29 and 30:
BOERHAAVE ON BLACK POWDER 29 Bertho
- Page 31 and 32:
BOERHAAVE ON BLACK POWDER 31 powder
- Page 33 and 34:
GREEK FIRE 33 And of the last two o
- Page 35 and 36:
ROGER BACON 35 only half filled wit
- Page 37 and 38:
ROGER BACON 37 large as the human t
- Page 39 and 40:
DEVELOPMENT OF BLACK POWDER 39 Deve
- Page 41 and 42:
DEVELOPMENT OF BLACK POWDER 41 the
- Page 43 and 44:
USES OF BLACK POWDER 43 Potassium n
- Page 45 and 46:
MANUFACTURE 45 rings and in other p
- Page 47 and 48:
ANALYSIS 47 caded, and is never app
- Page 49 and 50:
AMMONPULVER 49 ing is made from sod
- Page 51 and 52:
OTHER PROPELLENT EXPLOSIVES 51 toge
- Page 53 and 54:
PYROTECHNIC MIXTURES 53 colors. His
- Page 55 and 56:
PYROTECHNIC MIXTURES 55 composition
- Page 57 and 58:
PYROTECHNIC MIXTURES 57 and moisten
- Page 59 and 60:
PYROTECHNIC MIXTURES 59 chloride is
- Page 61 and 62:
PYROTECHNIC MIXTURES 61 positions w
- Page 63 and 64:
COLORED LIGHTS 63 flares during the
- Page 65 and 66:
Potassium chlorate Strontium nitrat
- Page 67 and 68:
Barium nitrate Potassium nitrate Po
- Page 69 and 70:
LANCES 69 minute with an illuminati
- Page 71 and 72:
PICRATE COMPOSITIONS 71 To be burne
- Page 73 and 74:
ROCKETS 73 is used in whistling fir
- Page 75 and 76:
ROCKETS 75 one ounce and a half. It
- Page 77 and 78:
ROCKETS 77 which handle a gross of
- Page 79 and 80:
Roman Candles ROMAN CANDLES 79 Roma
- Page 81 and 82:
STARS 81 the space between the star
- Page 83 and 84:
STARS 83 4-ply manila paper tubing,
- Page 85 and 86:
STARS 85 YVeingart 31 reports compo
- Page 87 and 88:
STARS 87 powder mixture, given a ye
- Page 89 and 90:
GERBS 89 Gerbs Gerbs produce jets o
- Page 91 and 92:
WHEELS 91 denly with a terrifying r
- Page 93 and 94:
PINWHEELS 93 Pinwheels To make pinw
- Page 95 and 96:
PINWHLELS 95 need of a vast theater
- Page 97 and 98:
MINES 97 match or fire wick), for t
- Page 99 and 100:
COMETS AND METEORS 99 an electric o
- Page 101 and 102:
BOMBSHELLS 101 the short pieces sep
- Page 103 and 104:
BOMBSHELLS 103 is folded, rubbed, a
- Page 105 and 106:
TOY CAPS 105 "It is to be noted," h
- Page 107 and 108:
JAPANESE TORPEDOES 107 minate but w
- Page 109 and 110:
RAILWAY TORPEDOES 109 hardens it),
- Page 111 and 112:
CHINESE FIRECRACKERS 111 English Cr
- Page 113 and 114:
CHINESE FIRECRACKERS 113 carrying o
- Page 115 and 116:
CHINESE FIRECRACKERS 115 struck a s
- Page 117 and 118:
SPARKLERS 117 is shaped by a motion
- Page 119 and 120:
PHARAOH'S SERPENTS 119 Wire Dips an
- Page 121 and 122:
BLACK NON-MERCURY SNAKES 121 pellet
- Page 123 and 124:
Potassium chlorate Lactose Paranitr
- Page 125 and 126:
CHAPTER IV AROMATIC NITRO COMPOUNDS
- Page 127 and 128:
EFFECT OF GROUPS ON FURTHER SUBSTIT
- Page 129 and 130:
UTILIZATION OF COAL TAR 129 the nuc
- Page 131 and 132:
UTILIZATION OF COAL TAR 131 In each
- Page 133 and 134:
MONO- AND DI-NITROBENZENE 133 Trini
- Page 135 and 136:
TRINITROBENZENE 135 powder, 250 lit
- Page 137 and 138:
TRINITROBENZENE 137 + CHsONo H. ,OC
- Page 139 and 140:
TRINITROBENZENE 139 According to Da
- Page 141 and 142:
TRINITROTOLUENE 141 not yet been de
- Page 143 and 144:
TRINITROTOLUENE 143 ?mall amount, p
- Page 145 and 146:
TRINITROTOLUENE 145 with a correspo
- Page 147 and 148:
TRINITROTOLUENE 14? The soluble sul
- Page 149 and 150:
TRINITROTOLUENE 149 cent free SOa)
- Page 151 and 152:
TRINITROTOLUENE 151 CH, NO, " ~" a
- Page 153 and 154:
TRIXITROXYLENE 153 Dautriche found
- Page 155 and 156:
NITRO DERIVATIVES OF NAPHTHALENE I5
- Page 157 and 158:
NITRO DERIVATIVES OF NAPHTHALENE 15
- Page 159 and 160:
PICRIC ACID 159 of biphenyl. The mo
- Page 161 and 162:
PICRIC ACID 161 dinitrophenol as re
- Page 163 and 164:
PICRIC ACID 163 a yellow nitropheno
- Page 165 and 166:
PICRIC ACID 165 below have been pub
- Page 167 and 168:
AMMONIUM PICRATE 167 80° and 90°
- Page 169 and 170:
TR1NITR0ANIS0L AND TRINITROPHENETOL
- Page 171 and 172:
TRINITROANISOL AND TRINITROPHENETOL
- Page 173 and 174:
TETRANITROANILINE 173 Both trinitro
- Page 175 and 176:
TETRYL 175 TNA shows about the same
- Page 177 and 178:
TETRYL 177 All the above-indicated
- Page 179 and 180:
TETRYL 179 m-nitrotetryl is effecti
- Page 181 and 182:
TETRYL 181 The solubility of tetryl
- Page 183 and 184:
BUTYL TETRYL 183 MINIMUM CHARGE FOB
- Page 185 and 186:
HEXANITRODIPHENYLAMINE 185 Carter 1
- Page 187 and 188:
HEXANITRODIPHENYL SULFONE 187 pound
- Page 189 and 190:
HEXANITROAZOBENZENE 189 nitrocarban
- Page 191 and 192:
CHAPTER V NITRIC ESTERS Nitric este
- Page 193 and 194:
METHYL NITRATE 193 sion of 24.5 mm.
- Page 195 and 196:
NITROGLYCERIN 195 blasting cap will
- Page 197 and 198:
NITROGLYCERIN 197 are evolved, but
- Page 199 and 200:
NITROGLYCERIN 199 than half its own
- Page 201 and 202:
NITROGLYCERIN 201 Thus, if 100 gram
- Page 203 and 204:
NITROGLYCERIN 203 quickly into a dr
- Page 205 and 206:
NITROGLYCERIN 205 "boils" strongly.
- Page 207 and 208:
NITROGLYCERIN 207 because the boili
- Page 209 and 210:
NITROGLYCERIN 209 the temperature r
- Page 211 and 212:
NITROGIA'CERIN 211 velocities of de
- Page 213 and 214:
NITROGLYCERIN 213 material in safet
- Page 215 and 216:
DINITROGLYCERIN 215 cerin. It mixes
- Page 217 and 218:
DINITROGLYCERIN 217 Both of the din
- Page 219 and 220:
NITROGLYCIDE 219 oxide ring, and mo
- Page 221 and 222:
DINITROCHLOROHYDRIN 221 can be froz
- Page 223 and 224:
NITROGLYCOL 223 amount is necessary
- Page 225 and 226:
NITROGLYCOL 225 portions with water
- Page 227 and 228:
TRINITROPHENOXYETHYL NITRATE 227 me
- Page 229 and 230:
PENTRYL 229 Another portion of the
- Page 231 and 232:
PENTRYL 231 tetryl by one of 27.5 c
- Page 233 and 234:
TRIMETHYLENE GLYCOL DINITRATE 233 i
- Page 235 and 236:
Butylene Glycol Dinitrate NITROERYT
- Page 237 and 238:
NITROMANNITE 23? While its stabilit
- Page 239 and 240:
NITRATED SUGAR MIXTURES 239 fact th
- Page 241 and 242:
NITROLACTOSE 241 Nitroglucose (d-Gl
- Page 243 and 244:
OTHER NITROSUGARS 243 readily solub
- Page 245 and 246:
EARLY HISTORY OF NITRATED CARBOHYDR
- Page 247 and 248:
EARLY HISTORY OF NITRATED CARBOHYDR
- Page 249 and 250:
EARLY HISTORY OF NITRATED CARBOHYDR
- Page 251 and 252:
EARLY HISTORY OF NITRATED CARBOHYDR
- Page 253 and 254:
EARLY HISTORY OF NITRATED CARBOHYDR
- Page 255 and 256:
EARLY HISTORY OF NITRATED CARBOHYDR
- Page 257 and 258:
NITROCELLULOSE 257 The two substanc
- Page 259 and 260:
NITROCELLULOSE 259 dre B. Either CP
- Page 261 and 262:
NITROCELLULOSE 261 nent materials w
- Page 263 and 264:
NltfROCELLULOSE 263 The pulped fibe
- Page 265 and 266:
NITROCELLULOSE 265 cellulose with g
- Page 267 and 268:
DURATION AT 1st period 2nd period 3
- Page 269 and 270:
DETERMINATION OF NITROGEN 269 upon
- Page 271 and 272:
DETERMINATION OF NITROGEN 271 actio
- Page 273 and 274:
NITROSTARCH 273 At the beginning of
- Page 275 and 276:
NJTROSTARCH 275 the contents of the
- Page 277 and 278:
UTILIZATION OF FORMALDEHYDE 277 cat
- Page 279 and 280:
PENTAERYTHRITE TETRANITRATE 279 ery
- Page 281 and 282:
DIPENTAERYTHRITE HEXANITRATE 281 ni
- Page 283 and 284:
TRIMETHYLOLNITROMETHANE TRINITRATE
- Page 285 and 286:
NITROPENTANONE AND RELATED SUBSTANC
- Page 287 and 288:
CHAPTER VI SMOKELESS POWDER An acco
- Page 289 and 290:
BULK POWDER 289 was called E C. pow
- Page 291 and 292:
BULK POWDER 291 FIGURE 71. Sweetie
- Page 293 and 294:
EARLY HISTORY OF COLLOIDED POWDERS
- Page 295 and 296:
EARLY HISTORY OF COLLOIDED POWDERS
- Page 297 and 298:
COLLOIDED NITROCELLULOSE POWDERS 29
- Page 299 and 300: MANUFACTURE OF SINGLE-BASE POWDER 2
- Page 301 and 302: MANUFACTURE OF SINGLE-BASE POWDER 3
- Page 303 and 304: MANUFACTURE OF SINGLE-BASE POWDER 3
- Page 305 and 306: MANUFACTURE OF SINGLE-BASE POWDER 3
- Page 307 and 308: STABILIZERS 307 Stabilizers The spo
- Page 309 and 310: STABILIZERS 309 stable as those con
- Page 311 and 312: TRANSFORMATIONS DURING AGING OF POW
- Page 313 and 314: ABSORPTION OF MOISTURE 313 ence is
- Page 315 and 316: ABSORPTION OF MOISTURE 315 ences. E
- Page 317 and 318: CONTROL OF RATE OF BURNING 317 GAIN
- Page 319 and 320: CONTROL OF RATE OF BURNING 319 Prog
- Page 321 and 322: GELATINIZING AGENTS 321 but very li
- Page 323 and 324: FLASHLESS CHARGES AND FLASHLESS POW
- Page 325 and 326: FLASHLESS CHARGES AND FLASHLESS POW
- Page 327 and 328: FLASHLESS CHARGES AND FLASHLESS POW
- Page 329 and 330: BALL-GRAIN POWDER 329 in the presen
- Page 331 and 332: CHAPTER VII DYNAMITE AND OTHER HIGH
- Page 333 and 334: INVENTION OF DYNAMITE 333 The next
- Page 335 and 336: INVENTION OF AMMONIUM NITRATE EXPLO
- Page 337 and 338: GUHR DYNAMITE 337 which is practica
- Page 339 and 340: STRAIGHT DYNAMITE 339 but "40% ammo
- Page 341 and 342: STRAIGHT DYNAMITE 341 Munroe and Ha
- Page 343 and 344: BLASTING GELATIN 343 of nitroglycer
- Page 345 and 346: GELATIN DYNAMITE 345 night, and the
- Page 347 and 348: PERMISSIBLE EXPLOSIVES 347 that one
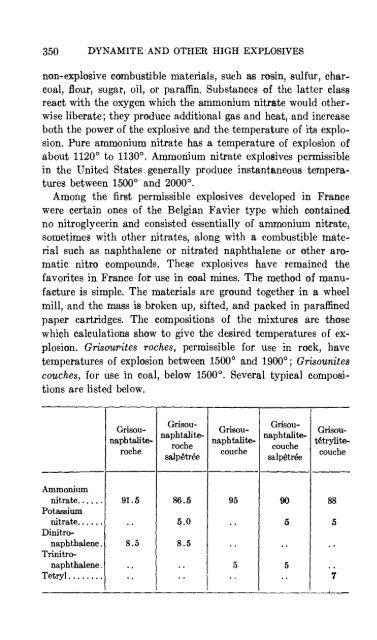
- Page 349: PERMISSIBLE EXPLOSIVES 349 many cla
- Page 353 and 354: Nitroglycenn Potassium nitrate Sodi
- Page 355 and 356: LIQUID OXYGEN EXPLOSIVES 355 The Pr
- Page 357 and 358: CHLORATE AND PERCHLORATE EXPLOSIVES
- Page 359 and 360: CHLORATE AND PERCHLORATE EXPLOSIVES
- Page 361 and 362: CHLORATE AND PERCHLORATE EXPLOSIVES
- Page 363 and 364: CHLORATE AND PERCHLORATE EXPLOSIVES
- Page 365 and 366: CHLORATE AND PERCHLORATE EXPLOSIVES
- Page 367 and 368: AMMONIUM NITRATE MILITARY EXPLOSIVE
- Page 369 and 370: CHAPTER VIII NITROAMINES AND RELATE
- Page 371 and 372: METHYLNITRAMINE 371 Methylnitramine
- Page 373 and 374: NITROUREA 373 filled with a strong
- Page 375 and 376: GUANIDINE NITRATE 375 Guanidine or
- Page 377 and 378: GUANIDINE NITRATE 377 The cyanamide
- Page 379 and 380: GUANIDINE NITRATE 379 only one mole
- Page 381 and 382: NITROGUANIDINE 381 been found, as d
- Page 383 and 384: NITROGUANIDINE 333 mixture, is trea
- Page 385 and 386: NITROGUANIDINE 385 A solution of ni
- Page 387 and 388: NITROGUANIDINE 387 Nitroguanidine,
- Page 389 and 390: NITROGUANIDINE 389 material "firmly
- Page 391 and 392: NITROSOGUANIDINE 391 stant volume o
- Page 393 and 394: ETHYLENEDINITRAMINE 393 rial which
- Page 395 and 396: DINITRODIMETHYLSULFAMIDE 395 gives
- Page 397 and 398: CVCLOTRIMETHVLENETRINITRAMINE 397 r
- Page 399 and 400: CYCLOTRIMETHYLENETRINITRAMINE 399 r
- Page 401 and 402:
DISCOVERY OF FULMINATING COMPOUNDS
- Page 403 and 404:
DISCOVERY OF FULMINATING COMPOUNDS
- Page 405 and 406:
MERCURY FULMINATE 405 cold anvil an
- Page 407 and 408:
MERCURY FULMINATE 407 water, filter
- Page 409 and 410:
MERCURY FULMINATE 409 HgSA + NaCN +
- Page 411 and 412:
MERCURY FULMINATE 411 mented with s
- Page 413 and 414:
DETONATORS 413 Amusements with Fulm
- Page 415 and 416:
DETONATORS 415 characters of the ch
- Page 417 and 418:
DETONATORS 417 potassium chlorate.
- Page 419 and 420:
DETONATORS 419 in the amount of san
- Page 421 and 422:
TESTING OF DETONATORS 421 pressed.
- Page 423 and 424:
TESTING OF DETONATORS 423 later rec
- Page 425 and 426:
LEAD AZIDE 425 used; the azide is e
- Page 427 and 428:
LEAD AZIDE 427 the interaction of h
- Page 429 and 430:
LEAD AZIDE 429 of which there exten
- Page 431 and 432:
SILVER AZIDE 431 temperature of spo
- Page 433 and 434:
CYANURIC TRIAZIDE 433 The best resu
- Page 435 and 436:
EXPLOSIVE Cyanuric triazide Lead az
- Page 437 and 438:
TRINITROTRIAZIDOBENZENE 437 purifie
- Page 439 and 440:
NITROGEN SULFIDE 439 acid, sulfur d
- Page 441 and 442:
DIAZONIUM SALTS 441 A 0.05-gram sam
- Page 443 and 444:
DIAZODINITROPHENOL 443 Preparation
- Page 445 and 446:
DIAZODINITROPHENOL 445 alcohol 2.43
- Page 447 and 448:
TETRACENE 447 its structure. It is
- Page 449 and 450:
TETRACENE 449 Preparation 0} Tetrac
- Page 451 and 452:
HEXAMETHYLENETRIPEROXIDEDIAMINE 451
- Page 453 and 454:
FRICTION PRIMERS 453 which had been
- Page 455 and 456:
PERCUSSION PRIMERS 455 or with wate
- Page 457 and 458:
PERCUSSION PRIMERS 457 is safest to
- Page 459 and 460:
Aaronson, 158, 226 Abel, 42, 194, 2
- Page 461 and 462:
Ge, 241 Geschwind, 151 Gibson, 127,
- Page 463 and 464:
Pliny, 35 Prentice and Co., 253 Pre
- Page 465 and 466:
INDEX OF SUBJECTS Alkylnitroguanidi
- Page 467 and 468:
Ballistite, attenuated, 259 erosive
- Page 469 and 470:
Carbon tetrabromide, 375 Carbon tet
- Page 471 and 472:
Dehydrating press, 299, 300, 302 De
- Page 473 and 474:
Ether, solvent, 135, 152, 169, 181,
- Page 475 and 476:
Gold, fulminating, 3, 400, 401 Gold
- Page 477 and 478:
K KI starch test, 268, 285 Kekule o
- Page 479 and 480:
Methylglucoside tetranitrate, 243 M
- Page 481 and 482:
Nitromaltose, 240, 241 Nitromannite
- Page 483 and 484:
Picric acid, Trauzl test, 211, 231
- Page 485 and 486:
Salt, common (sodium chloride), 60,
- Page 487 and 488:
Strength of dynamite, 338, 339, 341
- Page 489 and 490:
Trinitrobenzene, sensitivity to imp