Gas Laws
Gas Laws
Gas Laws
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The expression P/T k means that the result of pressure divided by<br />
temperature is a constant, k, for any sample of gas. If this is true, then<br />
P/T under one set of conditions is equal to P/T for the same sample of<br />
gas under another set of conditions, as long as the volume remains<br />
constant.<br />
Gay-Lussac’s law can be expressed by the following mathematical<br />
equation.<br />
124 Chapter 11<br />
P1 T1 P 2<br />
T 2<br />
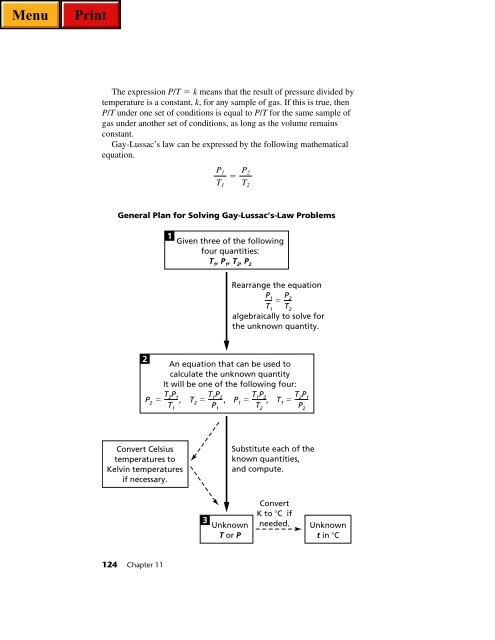
General Plan for Solving Gay-Lussac’s-Law Problems<br />
1<br />
Given three of the following<br />
four quantities:<br />
T 1 , P 1 , T 2 , P 2<br />
Rearrange the equation<br />
P1 T1 <br />
algebraically to solve for<br />
the unknown quantity.<br />
2<br />
An equation that can be used to<br />
calculate the unknown quantity<br />
It will be one of the following four:<br />
T2P1 P2 ,<br />
T1 T1P2 T2 ,<br />
P1 T1P2 P1 ,<br />
T2 T2P1 T1 <br />
P2 Convert Celsius<br />
temperatures to<br />
Kelvin temperatures<br />
if necessary.<br />
3<br />
Unknown<br />
T or P<br />
P2 T2 Substitute each of the<br />
known quantities,<br />
and compute.<br />
Convert<br />
K to C if<br />
needed.<br />
Unknown<br />
t in C