Mineral Industries and Geology of Certain Areas - Vermont Agency ...
Mineral Industries and Geology of Certain Areas - Vermont Agency ...
Mineral Industries and Geology of Certain Areas - Vermont Agency ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
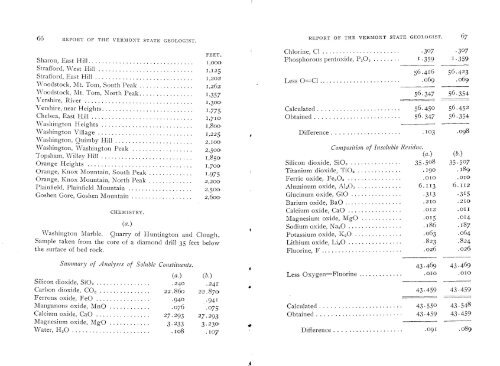
66 REPORT OF THE VERMONT STATE GEOLOGIST.<br />
REPORT OF THE VERMONT STATE GEOLOGIST. 67<br />
Chlorine, Cl ....................... .307 .307<br />
FEET<br />
Sharon, East Hill ...............................<br />
Phosphorous pentoxide, P 20 ........ I .359 I .359<br />
1,000,<br />
Strafford, West Hill 112 56.416 56.423<br />
Strafforci, East Hill<br />
I 2O_<br />
Less OCl 069 069<br />
\\ ooclstock, Mt Torn, South Peak 1,262<br />
Woodstock, Mt. Torn, North Peak ................ 1,357 56.347 56.354<br />
Vershire, River ................................ 1300<br />
Vershire, near Heights........................... 1.775 Calculated ........................ 56.450 56.452<br />
Chelsea, East Hill .............................. 1.710 Obtained ......................... 56.347 56.354<br />
\Vashington Heights ........................... i<br />
-<br />
800<br />
Washington Village ............................ 1.225 • Difference .................... . 103 .098<br />
Washington, Quimby Hill ....................... 2.100<br />
\Vashington, \Vashington Peak .................. 2.500 Composition <strong>of</strong> Insoluble Residue.<br />
Topsharn, Willey Hill ........................... (a.) (b.)<br />
i 80<br />
Orange Heights ............................... 1700<br />
Silicon dioxide, Si0 2 ............... 35.508 35.507<br />
Orange, Knox Mountain, South Peak Titanium dioxide, Ti0 2 ............. . 190 . 189<br />
Orange, Knox Mountain, North Peak ............. 2'2O0<br />
Plainfield. Plainfield Mountain ................... 2500<br />
Goshen Gore, Goshen Mountain .................. 2,600•<br />
Ferric oxide, Fe 203 ................ .010 .010<br />
Aluminum oxide, A1 20 .............. 6.113 6.112<br />
Glucinum oxide, G1O ............... .313 .315<br />
Barium oxide, BaO ................. .210 .210<br />
CHEMISTRY. Calcium oxide, CaO ................ .012 .011<br />
Magnesium oxide, MgO ............ .015 .014<br />
(a.)<br />
• Sodium oxide, Na 2O ................ . 186 .187<br />
Washington Marble. Quarry <strong>of</strong> Huntington <strong>and</strong> Clough Potassium oxide, K 20 .............. .063 .064<br />
Sample taken from the core <strong>of</strong> a diamond drill 35 feet below Ljthji.lrn oxide, Li 2O ................ .823 .824<br />
the surface <strong>of</strong> bed rock. Fluorine, F ........................ .026 .026<br />
Sunimary <strong>of</strong> Analyses <strong>of</strong> Soluble Constituents.<br />
•<br />
43 .469 43 .469<br />
(a.) (b.) Less Oxygen=Fluorine ............. .010 .010<br />
Silicon dioxide, SiO ................. .240 .24[<br />
Carbon dioxide, CO 2 ...............22.86o 22.870 • .459 43 .459<br />
Ferrous oxide, FeO .................940 .941<br />
Manganous oxide, MnO .............076 .075; Calculated ........................ 43.55 0 43.548<br />
Calcium oxide, CaO ................27.293 27.293; Obtained ......................... 43.459 43.459<br />
Magnesium oxide, MgO ............3.233 3.230<br />
Water, H 20 ........................ 108 .107 Difference .................... .091 .089