Management of pregnancy - VA/DoD Clinical Practice Guidelines ...
Management of pregnancy - VA/DoD Clinical Practice Guidelines ...
Management of pregnancy - VA/DoD Clinical Practice Guidelines ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>VA</strong>/<strong>DoD</strong> <strong>Clinical</strong> <strong>Practice</strong> Guideline<br />
For Pregnancy <strong>Management</strong><br />
Interventions Not Recommended in Prenatal Care (All Weeks)<br />
I 52. Routine Screening with Fetal Fibronectin: Not Recommended<br />
BACKGROUND<br />
Fetal fibronectin levels can identify pregnant women at risk for preterm delivery. Routine fetal fibronectin<br />
screening <strong>of</strong> cervical vaginal fluid has been suggested by some experts as a means <strong>of</strong> reducing preterm delivery<br />
among low-risk/asymptomatic pregnancies. However, there is insufficient data to support fetal fibronectin screening<br />
in all pregnant women.<br />
RECOMMENDATIONS<br />
1.<br />
2.<br />
Recommend against routine screening for preterm birth with fetal fibronectin (fFN) test. [D]<br />
Utilization <strong>of</strong> fFN testing in symptomatic women between 24 and 34 6/7 weeks’ gestation may be useful in<br />
guiding management <strong>of</strong> women with signs and symptoms <strong>of</strong> preterm labor. [B]<br />
DISCUSSION<br />
In a large meta-analysis, the accuracy for predicting spontaneous preterm birth using the fFN test varied<br />
considerably with no significant differences in estimates <strong>of</strong> accuracy in studies with high/low quality (Honest et al,<br />
2002). Several prospective cohort studies have shown no improvement in outcomes for either mother or baby<br />
(Leitech et al., 1999; Ramsey & Andrews, 2003). The routine use <strong>of</strong> this expensive technology is not justified in<br />
light <strong>of</strong> the low predictive value <strong>of</strong> either a positive or negative test, along with absence <strong>of</strong> an effective intervention.<br />
The potential value <strong>of</strong> fFN testing in the setting <strong>of</strong> questionable preterm labor is to more precisely discriminate<br />
between the subset <strong>of</strong> women who have true preterm labor versus false labor. A negative test in women with<br />
preterm contractions may provide information sufficient to avoid the use <strong>of</strong> tocolytics and corticosteroids in an<br />
individual at low risk for preterm birth (Ramsey & Andrews, 2003).<br />
The following was noted in a memorandum reporting the findings and recommendations <strong>of</strong> a multidisciplinary and<br />
multi-service national Department <strong>of</strong> Defense (<strong>DoD</strong>) committee regarding the use <strong>of</strong> fFN testing as an adjunct to the<br />
assessment <strong>of</strong> suspected preterm labor: the primary benefit [<strong>of</strong> using the fFN test] is in patients presenting with<br />
preterm contractions where [one is] clinically uncertain if the patient will be delivering within one to two weeks.<br />
The fFN specimen should only be sent if the result would potentially change the patient’s management. It should<br />
not be used if it will not alter care (<strong>DoD</strong> Committee Consensus on Fetal Fibronectin Testing in Pregnancy (2007).<br />
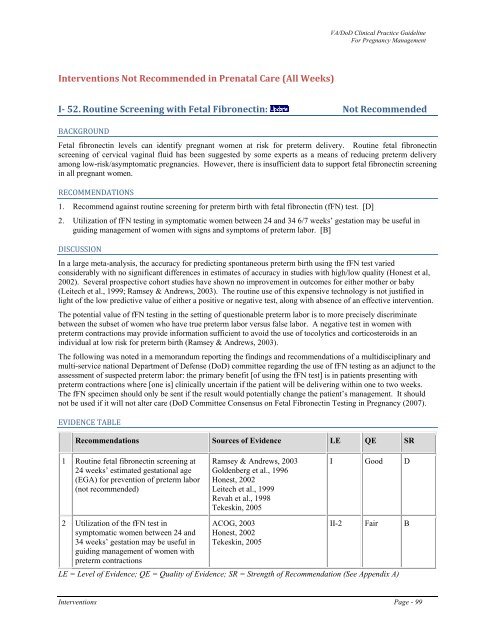
EVIDENCE TABLE<br />
Recommendations Sources <strong>of</strong> Evidence LE QE SR<br />
1 Routine fetal fibronectin screening at<br />
24 weeks’ estimated gestational age<br />
(EGA) for prevention <strong>of</strong> preterm labor<br />
(not recommended)<br />
Ramsey & Andrews, 2003<br />
Goldenberg et al., 1996<br />
Honest, 2002<br />
Leitech et al., 1999<br />
Revah et al., 1998<br />
Tekeskin, 2005<br />
I Good D<br />
2 Utilization <strong>of</strong> the fFN test in<br />
symptomatic women between 24 and<br />
34 weeks’ gestation may be useful in<br />
guiding management <strong>of</strong> women with<br />
preterm contractions<br />
ACOG, 2003<br />
Honest, 2002<br />
Tekeskin, 2005<br />
II-2 Fair B<br />
LE = Level <strong>of</strong> Evidence; QE = Quality <strong>of</strong> Evidence; SR = Strength <strong>of</strong> Recommendation (See Appendix A)<br />
Interventions Page - 99