Management of pregnancy - VA/DoD Clinical Practice Guidelines ...
Management of pregnancy - VA/DoD Clinical Practice Guidelines ...
Management of pregnancy - VA/DoD Clinical Practice Guidelines ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>VA</strong>/<strong>DoD</strong> <strong>Clinical</strong> <strong>Practice</strong> Guideline<br />
For Pregnancy <strong>Management</strong><br />
at least two values <strong>of</strong> MSAFP exceeding 2.5 MOM, when corrected for gestational age, should be referred to<br />
advanced prenatal care follow-up.<br />
Down syndrome (trisomy 21) occurs in 1/800 births, increasing in risk with advancing maternal age. Eighty percent<br />
<strong>of</strong> babies with Down syndrome are born to women under 35 with no risk factors. Low MSAFP is associated with<br />
increased risk for Down syndrome (Haddow et al., 1992). If risk for Down syndrome is calculated solely on age<br />
versus AFP, detection increases from 25 to 37 percent. Pregnant women with fetuses affected by trisomy 21 tend to<br />
have lower than average levels <strong>of</strong> MSAFP and unconjugated estriol with elevated levels <strong>of</strong> serum HCG, when<br />
compared to women carrying euploid fetuses. Adding serum HCG and uncongugated estriol (“triple screen”)<br />
increases detection to 56 to 75 percent without increasing false positivity (Smith-Bindman et al., 2001). Triple<br />
screen also increases the antenatal detection rate for a variety <strong>of</strong> chromosome disorders, particularly sex<br />
chromosome abnormalities (Kellner et al., 1995). Ultrasound to assess fetal age is indicated for all women with low<br />
MSAFP or abnormal triple screen. It should be followed by amniocentesis for gestational age-adjusted persistent<br />
abnormal values. The benefit <strong>of</strong> increased detection <strong>of</strong> chromosome abnormalities should be weighed against the<br />
risk <strong>of</strong> fetal loss from amniocentesis.<br />
Edward’s Syndrome (trisomy 18) occurs in approximately one in 5,000 live births and is associated with a high rate<br />
<strong>of</strong> fetal death or early neonatal demise. Affected individuals surviving the neonatal period typically have pr<strong>of</strong>ound<br />
neurodevelopmental delay and are unlikely to survive beyond five years <strong>of</strong> age. Pregnant women with fetuses<br />
affected by trisomy 18 tend to have lower than average levels <strong>of</strong> MSAFP, HCG, and unconjugated estriol.<br />
Approximately 50 percent <strong>of</strong> fetuses with trisomy 18 can be detected with maternal serum analyte screening and<br />
follow-up fetal karyotype analysis <strong>of</strong> screen-positive women.<br />
Customary practice is to <strong>of</strong>fer amniocentesis or chorionic villus sampling to all women age 35 or older at the time <strong>of</strong><br />
birth, and to women whose risk <strong>of</strong> Down syndrome by maternal serum analyte screening is equivalent to that <strong>of</strong> a<br />
35-year-old woman. Data from randomized trials suggest the attributable risk <strong>of</strong> <strong>pregnancy</strong> loss due to<br />
amniocentesis is less than 1:1500 when performed by experienced clinicians. Accordingly, ACOG has<br />
recommended amniocentesis be available to women <strong>of</strong> all ages regardless <strong>of</strong> their a priori risk for carrying a fetus<br />
with aneuploidy (ACOG, 2007).<br />
For gravidas over 35, maternal serum analyte screening with subsequent confirmatory fetal karyotype analysis <strong>of</strong><br />
screen-positive women identify up to 89 percent <strong>of</strong> fetuses with Down syndrome, with a false positive rate <strong>of</strong> 25<br />
percent. For pregnant women over 35 who are willing to accept a potentially false-negative screen, the quad screen<br />
is a cost effective alternative to routine amniocentesis. This alternative practice could make more than 75 percent <strong>of</strong><br />
amniocenteses unnecessary, thereby also reducing amniocentesis-associated fetal losses (Haddow et al., 1994). The<br />
complexity <strong>of</strong> the pre-screening and pre-testing counseling requires referral <strong>of</strong> high-risk women to a qualified<br />
healthcare provider for counseling. Any pregnant women determined to have a fetus with a serious structural<br />
abnormality or fetal aneuploidy should receive advanced prenatal care.<br />
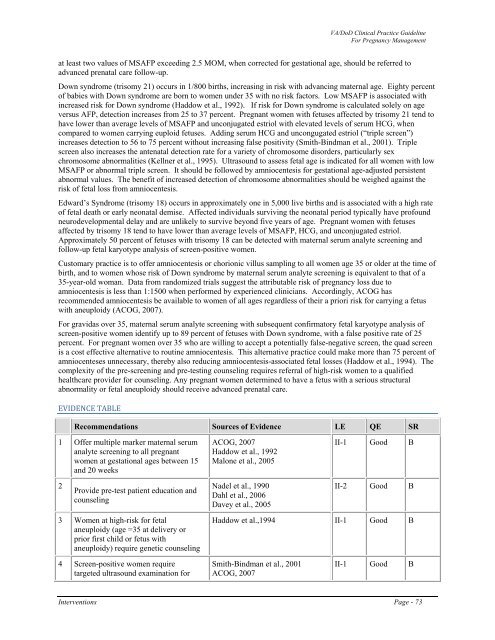
EVIDENCE TABLE<br />
Recommendations Sources <strong>of</strong> Evidence LE QE SR<br />
1 Offer multiple marker maternal serum<br />
analyte screening to all pregnant<br />
women at gestational ages between 15<br />
and 20 weeks<br />
ACOG, 2007<br />
Haddow et al., 1992<br />
Malone et al., 2005<br />
II-1 Good B<br />
2<br />
Provide pre-test patient education and<br />
counseling<br />
Nadel et al., 1990<br />
Dahl et al., 2006<br />
Davey et al., 2005<br />
II-2 Good B<br />
3 Women at high-risk for fetal<br />
aneuploidy (age =35 at delivery or<br />
prior first child or fetus with<br />
aneuploidy) require genetic counseling<br />
Haddow et al.,1994 II-1 Good B<br />
4 Screen-positive women require<br />
targeted ultrasound examination for<br />
Smith-Bindman et al., 2001<br />
ACOG, 2007<br />
II-1 Good B<br />
Interventions Page - 73