Management of pregnancy - VA/DoD Clinical Practice Guidelines ...
Management of pregnancy - VA/DoD Clinical Practice Guidelines ...
Management of pregnancy - VA/DoD Clinical Practice Guidelines ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>DoD</strong>/<strong>VA</strong> <strong>Clinical</strong> <strong>Practice</strong> Guideline for<br />
Pregnancy <strong>Management</strong><br />
B1<br />
B2<br />
D1<br />
D2<br />
D3<br />
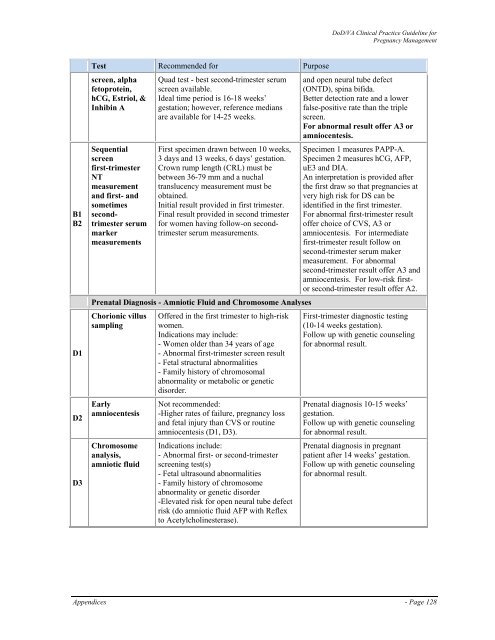
Test Recommended for Purpose<br />
screen, alpha<br />
fetoprotein,<br />
hCG, Estriol, &<br />
Inhibin A<br />
Sequential<br />
screen<br />
first-trimester<br />
NT<br />
measurement<br />
and first- and<br />
sometimes<br />
secondtrimester<br />
serum<br />
marker<br />
measurements<br />
Quad test - best second-trimester serum<br />
screen available.<br />
Ideal time period is 16-18 weeks’<br />
gestation; however, reference medians<br />
are available for 14-25 weeks.<br />
First specimen drawn between 10 weeks,<br />
3 days and 13 weeks, 6 days’ gestation.<br />
Crown rump length (CRL) must be<br />
between 36-79 mm and a nuchal<br />
translucency measurement must be<br />
obtained.<br />
Initial result provided in first trimester.<br />
Final result provided in second trimester<br />
for women having follow-on secondtrimester<br />
serum measurements.<br />
Prenatal Diagnosis - Amniotic Fluid and Chromosome Analyses<br />
Chorionic villus<br />
sampling<br />
Early<br />
amniocentesis<br />
Chromosome<br />
analysis,<br />
amniotic fluid<br />
Offered in the first trimester to high-risk<br />
women.<br />
Indications may include:<br />
- Women older than 34 years <strong>of</strong> age<br />
- Abnormal first-trimester screen result<br />
- Fetal structural abnormalities<br />
- Family history <strong>of</strong> chromosomal<br />
abnormality or metabolic or genetic<br />
disorder.<br />
Not recommended:<br />
-Higher rates <strong>of</strong> failure, <strong>pregnancy</strong> loss<br />
and fetal injury than CVS or routine<br />
amniocentesis (D1, D3).<br />
Indications include:<br />
- Abnormal first- or second-trimester<br />
screening test(s)<br />
- Fetal ultrasound abnormalities<br />
- Family history <strong>of</strong> chromosome<br />
abnormality or genetic disorder<br />
-Elevated risk for open neural tube defect<br />
risk (do amniotic fluid AFP with Reflex<br />
to Acetylcholinesterase).<br />
and open neural tube defect<br />
(ONTD), spina bifida.<br />
Better detection rate and a lower<br />
false-positive rate than the triple<br />
screen.<br />
For abnormal result <strong>of</strong>fer A3 or<br />
amniocentesis.<br />
Specimen 1 measures PAPP-A.<br />
Specimen 2 measures hCG, AFP,<br />
uE3 and DIA.<br />
An interpretation is provided after<br />
the first draw so that pregnancies at<br />
very high risk for DS can be<br />
identified in the first trimester.<br />
For abnormal first-trimester result<br />
<strong>of</strong>fer choice <strong>of</strong> CVS, A3 or<br />
amniocentesis. For intermediate<br />
first-trimester result follow on<br />
second-trimester serum maker<br />
measurement. For abnormal<br />
second-trimester result <strong>of</strong>fer A3 and<br />
amniocentesis. For low-risk firstor<br />
second-trimester result <strong>of</strong>fer A2.<br />
First-trimester diagnostic testing<br />
(10-14 weeks gestation).<br />
Follow up with genetic counseling<br />
for abnormal result.<br />
Prenatal diagnosis 10-15 weeks’<br />
gestation.<br />
Follow up with genetic counseling<br />
for abnormal result.<br />
Prenatal diagnosis in pregnant<br />
patient after 14 weeks’ gestation.<br />
Follow up with genetic counseling<br />
for abnormal result.<br />
Appendices - Page 128