CDC Article-US Medical Eligibility Criteria for Contraceptive Use, 2010
CDC Article-US Medical Eligibility Criteria for Contraceptive Use, 2010
CDC Article-US Medical Eligibility Criteria for Contraceptive Use, 2010
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
38 MMWR June 18, <strong>2010</strong><br />
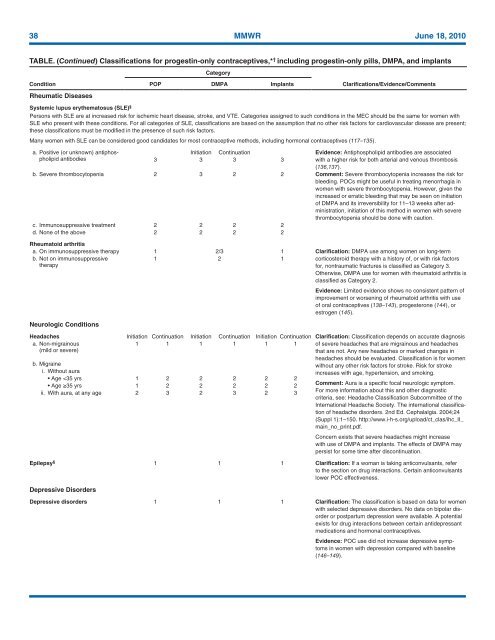
TABLE. (Continued) Classifications <strong>for</strong> progestin-only contraceptives,* † including progestin-only pills, DMPA, and implants<br />
Category<br />
Condition<br />
Rheumatic Diseases<br />
POP DMPA Implants<br />
Clarifications/Evidence/Comments<br />
Systemic lupus erythematosus (SLE) §<br />
Persons with SLE are at increased risk <strong>for</strong> ischemic heart disease, stroke, and VTE. Categories assigned to such conditions in the MEC should be the same <strong>for</strong> women with<br />
SLE who present with these conditions. For all categories of SLE, classifications are based on the assumption that no other risk factors <strong>for</strong> cardiovascular disease are present;<br />
these classifications must be modified in the presence of such risk factors.<br />
Many women with SLE can be considered good candidates <strong>for</strong> most contraceptive methods, including hormonal contraceptives (117–135).<br />
a. Positive (or unknown) antiphospholipid<br />
Initiation Continuation Evidence: Antiphospholipid antibodies are associated<br />
antibodies<br />
3 3 3 3<br />
with a higher risk <strong>for</strong> both arterial and venous thrombosis<br />
(136,137).<br />
b. Severe thrombocytopenia 2 3 2 2 Comment: Severe thrombocytopenia increases the risk <strong>for</strong><br />
bleeding. POCs might be useful in treating menorrhagia in<br />
women with severe thrombocytopenia. However, given the<br />
increased or erratic bleeding that may be seen on initiation<br />
of DMPA and its irreversibility <strong>for</strong> 11–13 weeks after administration,<br />
initiation of this method in women with severe<br />
thrombocytopenia should be done with caution.<br />
c. Immunosuppressive treatment 2 2 2 2<br />
d. None of the above 2 2 2 2<br />
Rheumatoid arthritis<br />
a. On immunosuppressive therapy 1 2/3 1 Clarification: DMPA use among women on long-term<br />
b. Not on immunosuppressive<br />
therapy<br />
1 2 1<br />
corticosteroid therapy with a history of, or with risk factors<br />
<strong>for</strong>, nontraumatic fractures is classified as Category 3.<br />
Otherwise, DMPA use <strong>for</strong> women with rheumatoid arthritis is<br />
classified as Category 2.<br />
Evidence: Limited evidence shows no consistent pattern of<br />
improvement or worsening of rheumatoid arthritis with use<br />
of oral contraceptives (138–143), progesterone (144), or<br />
estrogen (145).<br />
Neurologic Conditions<br />
Headaches Initiation Continuation Initiation Continuation Initiation Continuation Clarification: Classification depends on accurate diagnosis<br />
a. Non-migrainous<br />
(mild or severe)<br />
1 1 1 1 1 1 of severe headaches that are migrainous and headaches<br />
that are not. Any new headaches or marked changes in<br />
headaches should be evaluated. Classification is <strong>for</strong> women<br />
without any other risk factors <strong>for</strong> stroke. Risk <strong>for</strong> stroke<br />
increases with age, hypertension, and smoking.<br />
b. Migraine<br />
i. Without aura<br />
• Age