CDC Article-US Medical Eligibility Criteria for Contraceptive Use, 2010
CDC Article-US Medical Eligibility Criteria for Contraceptive Use, 2010
CDC Article-US Medical Eligibility Criteria for Contraceptive Use, 2010
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
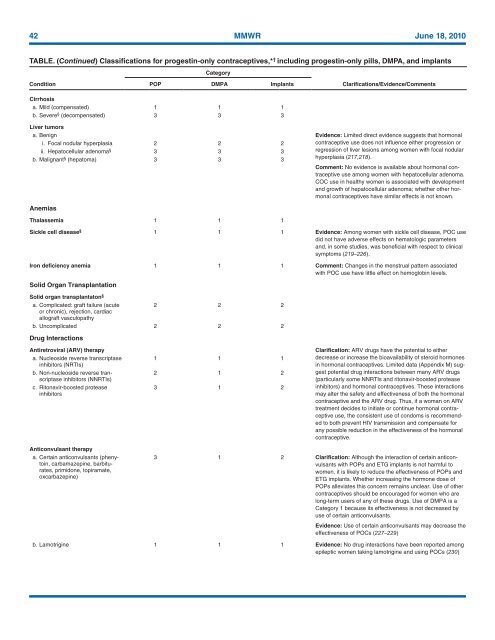
42 MMWR June 18, <strong>2010</strong><br />
TABLE. (Continued) Classifications <strong>for</strong> progestin-only contraceptives,* † including progestin-only pills, DMPA, and implants<br />
Category<br />
Condition<br />
POP DMPA Implants<br />
Clarifications/Evidence/Comments<br />
Cirrhosis<br />
a. Mild (compensated) 1 1 1<br />
b. Severe § (decompensated) 3 3 3<br />
Liver tumors<br />
a. Benign Evidence: Limited direct evidence suggests that hormonal<br />
i. Focal nodular hyperplasia 2 2 2<br />
ii. Hepatocellular adenoma § 3 3 3<br />
b. Malignant § (hepatoma) 3 3 3<br />
Anemias<br />
Thalassemia 1 1 1<br />
contraceptive use does not influence either progression or<br />
regression of liver lesions among women with focal nodular<br />
hyperplasia (217,218).<br />
Comment: No evidence is available about hormonal contraceptive<br />
use among women with hepatocellular adenoma.<br />
COC use in healthy women is associated with development<br />
and growth of hepatocellular adenoma; whether other hormonal<br />
contraceptives have similar effects is not known.<br />
Sickle cell disease § 1 1 1 Evidence: Among women with sickle cell disease, POC use<br />
did not have adverse effects on hematologic parameters<br />
and, in some studies, was beneficial with respect to clinical<br />
symptoms (219–226).<br />
Iron deficiency anemia 1 1 1 Comment: Changes in the menstrual pattern associated<br />
with POC use have little effect on hemoglobin levels.<br />
Solid Organ Transplantation<br />
Solid organ transplantaton §<br />
a. Complicated: graft failure (acute<br />
2 2 2<br />
or chronic), rejection, cardiac<br />
allograft vasculopathy<br />
b. Uncomplicated 2 2 2<br />
Drug Interactions<br />
Antiretroviral (ARV) therapy<br />
a. Nucleoside reverse transcriptase<br />
inhibitors (NRTIs)<br />
b. Non-nucleoside reverse transcriptase<br />
inhibitors (NNRTIs)<br />
c. Ritonavir-boosted protease<br />
inhibitors<br />
1 1 1<br />
2 1 2<br />
3 1 2<br />
Clarification: ARV drugs have the potential to either<br />
decrease or increase the bioavailability of steroid hormones<br />
in hormonal contraceptives. Limited data (Appendix M) suggest<br />
potential drug interactions between many ARV drugs<br />
(particularly some NNRTIs and ritonavir-boosted protease<br />
inhibitors) and hormonal contraceptives. These interactions<br />
may alter the safety and effectiveness of both the hormonal<br />
contraceptive and the ARV drug. Thus, if a woman on ARV<br />
treatment decides to initiate or continue hormonal contraceptive<br />
use, the consistent use of condoms is recommended<br />
to both prevent HIV transmission and compensate <strong>for</strong><br />
any possible reduction in the effectiveness of the hormonal<br />
contraceptive.<br />
Anticonvulsant therapy<br />
a. Certain anticonvulsants (phenytoin,<br />
carbamazepine, barbiturates,<br />
primidone, topiramate,<br />
oxcarbazepine)<br />
3 1 2 Clarification: Although the interaction of certain anticonvulsants<br />
with POPs and ETG implants is not harmful to<br />
women, it is likely to reduce the effectiveness of POPs and<br />
ETG implants. Whether increasing the hormone dose of<br />
POPs alleviates this concern remains unclear. <strong>Use</strong> of other<br />
contraceptives should be encouraged <strong>for</strong> women who are<br />
long-term users of any of these drugs. <strong>Use</strong> of DMPA is a<br />
Category 1 because its effectiveness is not decreased by<br />
use of certain anticonvulsants.<br />
Evidence: <strong>Use</strong> of certain anticonvulsants may decrease the<br />
effectiveness of POCs (227–229)<br />
b. Lamotrigine 1 1 1 Evidence: No drug interactions have been reported among<br />
epileptic women taking lamotrigine and using POCs (230)