CDC Article-US Medical Eligibility Criteria for Contraceptive Use, 2010
CDC Article-US Medical Eligibility Criteria for Contraceptive Use, 2010
CDC Article-US Medical Eligibility Criteria for Contraceptive Use, 2010
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
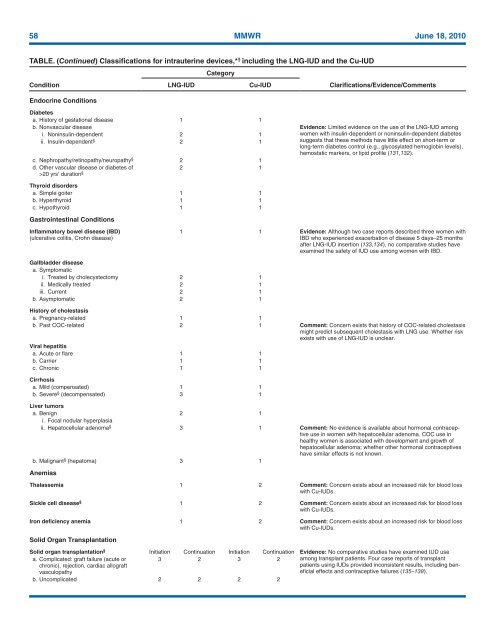
58 MMWR June 18, <strong>2010</strong><br />
TABLE. (Continued) Classifications <strong>for</strong> intrauterine devices,* † including the LNG-IUD and the Cu-IUD<br />
Condition<br />
Endocrine Conditions<br />
LNG-IUD<br />
Category<br />
Cu-IUD<br />
Clarifications/Evidence/Comments<br />
Diabetes<br />
a. History of gestational disease 1 1<br />
b. Nonvascular disease Evidence: Limited evidence on the use of the LNG-IUD among<br />
i. Noninsulin-dependent 2 1<br />
ii. Insulin-dependent § 2 1<br />
c. Nephropathy/retinopathy/neuropathy § 2 1<br />
d. Other vascular disease or diabetes of<br />
2 1<br />
>20 yrs’ duration §<br />
Thyroid disorders<br />
a. Simple goiter 1 1<br />
b. Hyperthyroid 1 1<br />
c. Hypothyroid 1 1<br />
Gastrointestinal Conditions<br />
women with insulin-dependent or noninsulin-dependent diabetes<br />
suggests that these methods have little effect on short-term or<br />
long-term diabetes control (e.g., glycosylated hemoglobin levels),<br />
hemostatic markers, or lipid profile (131,132).<br />
Inflammatory bowel disease (IBD)<br />
(ulcerative colitis, Crohn disease)<br />
1 1 Evidence: Although two case reports described three women with<br />
IBD who experienced exacerbation of disease 5 days–25 months<br />
after LNG-IUD insertion (133,134), no comparative studies have<br />
examined the safety of IUD use among women with IBD.<br />
Gallbladder disease<br />
a. Symptomatic<br />
i. Treated by cholecystectomy 2 1<br />
ii. <strong>Medical</strong>ly treated 2 1<br />
iii. Current 2 1<br />
b. Asymptomatic 2 1<br />
History of cholestasis<br />
a. Pregnancy-related 1 1<br />
b. Past COC-related 2 1 Comment: Concern exists that history of COC-related cholestasis<br />
might predict subsequent cholestasis with LNG use. Whether risk<br />
exists with use of LNG-IUD is unclear.<br />
Viral hepatitis<br />
a. Acute or flare 1 1<br />
b. Carrier 1 1<br />
c. Chronic 1 1<br />
Cirrhosis<br />
a. Mild (compensated) 1 1<br />
b. Severe § (decompensated) 3 1<br />
Liver tumors<br />
a. Benign 2 1<br />
i. Focal nodular hyperplasia<br />
ii. Hepatocellular adenoma § 3 1 Comment: No evidence is available about hormonal contraceptive<br />
use in women with hepatocellular adenoma. COC use in<br />
healthy women is associated with development and growth of<br />
hepatocellular adenoma; whether other hormonal contraceptives<br />
have similar effects is not known.<br />
b. Malignant § (hepatoma) 3 1<br />
Anemias<br />
Thalassemia 1 2 Comment: Concern exists about an increased risk <strong>for</strong> blood loss<br />
with Cu-IUDs.<br />
Sickle cell disease § 1 2 Comment: Concern exists about an increased risk <strong>for</strong> blood loss<br />
with Cu-IUDs.<br />
Iron deficiency anemia 1 2 Comment: Concern exists about an increased risk <strong>for</strong> blood loss<br />
with Cu-IUDs.<br />
Solid Organ Transplantation<br />
Solid organ transplantation § Initiation Continuation Initiation Continuation Evidence: No comparative studies have examined IUD use<br />
a. Complicated: graft failure (acute or<br />
3 2 3 2 among transplant patients. Four case reports of transplant<br />
chronic), rejection, cardiac allograft<br />
vasculopathy<br />
patients using IUDs provided inconsistent results, including beneficial<br />
effects and contraceptive failures (135–138).<br />
b. Uncomplicated 2 2 2 2