CDC Article-US Medical Eligibility Criteria for Contraceptive Use, 2010
CDC Article-US Medical Eligibility Criteria for Contraceptive Use, 2010
CDC Article-US Medical Eligibility Criteria for Contraceptive Use, 2010
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
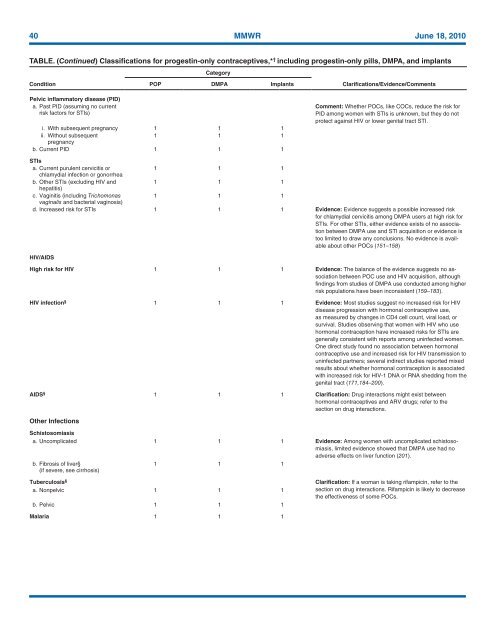
40 MMWR June 18, <strong>2010</strong><br />
TABLE. (Continued) Classifications <strong>for</strong> progestin-only contraceptives,* † including progestin-only pills, DMPA, and implants<br />
Category<br />
Condition<br />
POP DMPA Implants<br />
Clarifications/Evidence/Comments<br />
Pelvic inflammatory disease (PID)<br />
a. Past PID (assuming no current<br />
risk factors <strong>for</strong> STIs)<br />
i. With subsequent pregnancy 1 1 1<br />
ii. Without subsequent<br />
1 1 1<br />
pregnancy<br />
b. Current PID 1 1 1<br />
Comment: Whether POCs, like COCs, reduce the risk <strong>for</strong><br />
PID among women with STIs is unknown, but they do not<br />
protect against HIV or lower genital tract STI.<br />
STIs<br />
a. Current purulent cervicitis or<br />
1 1 1<br />
chlamydial infection or gonorrhea<br />
b. Other STIs (excluding HIV and<br />
1 1 1<br />
hepatitis)<br />
c. Vaginitis (including Trichomonas<br />
1 1 1<br />
vaginalis and bacterial vaginosis)<br />
d. Increased risk <strong>for</strong> STIs 1 1 1 Evidence: Evidence suggests a possible increased risk<br />
<strong>for</strong> chlamydial cervicitis among DMPA users at high risk <strong>for</strong><br />
STIs. For other STIs, either evidence exists of no association<br />
between DMPA use and STI acquisition or evidence is<br />
too limited to draw any conclusions. No evidence is available<br />
about other POCs (151–158)<br />
HIV/AIDS<br />
High risk <strong>for</strong> HIV 1 1 1 Evidence: The balance of the evidence suggests no association<br />
between POC use and HIV acquisition, although<br />
findings from studies of DMPA use conducted among higher<br />
risk populations have been inconsistent (159–183).<br />
HIV infection § 1 1 1 Evidence: Most studies suggest no increased risk <strong>for</strong> HIV<br />
disease progression with hormonal contraceptive use,<br />
as measured by changes in CD4 cell count, viral load, or<br />
survival. Studies observing that women with HIV who use<br />
hormonal contraception have increased risks <strong>for</strong> STIs are<br />
generally consistent with reports among uninfected women.<br />
One direct study found no association between hormonal<br />
contraceptive use and increased risk <strong>for</strong> HIV transmission to<br />
uninfected partners; several indirect studies reported mixed<br />
results about whether hormonal contraception is associated<br />
with increased risk <strong>for</strong> HIV-1 DNA or RNA shedding from the<br />
genital tract (171,184–200).<br />
AIDS § 1 1 1 Clarification: Drug interactions might exist between<br />
hormonal contraceptives and ARV drugs; refer to the<br />
section on drug interactions.<br />
Other Infections<br />
Schistosomiasis<br />
a. Uncomplicated 1 1 1 Evidence: Among women with uncomplicated schistosomiasis,<br />
limited evidence showed that DMPA use had no<br />
adverse effects on liver function (201).<br />
b. Fibrosis of liver§<br />
(if severe, see cirrhosis)<br />
1 1 1<br />
Tuberculosis §<br />
a. Nonpelvic 1 1 1<br />
b. Pelvic 1 1 1<br />
Clarification: If a woman is taking rifampicin, refer to the<br />
section on drug interactions. Rifampicin is likely to decrease<br />
the effectiveness of some POCs.<br />
Malaria 1 1 1