2012 Corporate Capabilities - Spectroscopy
2012 Corporate Capabilities - Spectroscopy
2012 Corporate Capabilities - Spectroscopy
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
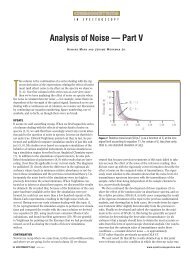
14 <strong>Spectroscopy</strong> 26(12) December 2011 www.spectroscopyonline.com<br />
Focus on Quality<br />
USP and the<br />
GAMP Guide on Laboratory<br />
Computerized Systems — Is<br />
Integration Possible?<br />
The United States Pharmacopeia general chapter on analytical instrument qualification (USP<br />
) and the ISPE’s Good Automated Manufacturing Practice (GAMP) Good Practice Guide<br />
on laboratory computerized systems are the two main sources of guidance for qualifying analytical<br />
instruments and validating computerized systems used in the laboratory. This column<br />
explains the discrepancies between the two documents as well as changes now being made to<br />
both in an attempt to enable an integrated approach to qualification and validation of laboratory<br />
instruments and systems.<br />
R.D. McDowall and C. Burgess<br />
There are many sources of advice on computerized<br />
system validation and analytical instrument qualification<br />
for the laboratory, including regulatory<br />
agencies, such as the United States Food and Drug Administration<br />
(FDA) (1,2); regulatory associations such as<br />
the Pharmaceutical Inspection Convention/Co-operation<br />
Scheme (PIC/S) (3,4); the Official Medicines Control<br />
Laboratories (OMCL) in Europe (5); and pharmacopeias<br />
such as the United States Pharmacopeia (USP) (6). Information<br />
also can be obtained from scientific societies or<br />
associations such as the American Association of Pharmaceutical<br />
Scientists (AAPS) (7), the Parenteral Drug<br />
Association (PDA) (8), the Drug Information Association<br />
(DIA) (9), and the International Society of Pharmaceutical<br />
Engineering (ISPE) (10). All of these organizations<br />
have published guidance on instrument qualification or<br />
computer validation either for a general regulated audience<br />
or specifically for a regulated laboratory.<br />
There are two main sources, however, of regulatory<br />
guidance and advice for qualification of analytical instruments<br />
and validation of computerized systems used in<br />
the laboratory. The first is USP general chapter on<br />
analytical instrument qualification (AIQ) (6), which was<br />
derived from an AAPS meeting on analytical instrument<br />
validation held in 2003. One decision that came from that<br />
conference was that the terminology being used at the time<br />
was incorrect, because the conference name itself should<br />
have referred to analytical instrument qualification. The<br />
white paper published by AAPS in 2004 (7) was the major<br />
input to USP , which became effective in 2008.