2012 Corporate Capabilities - Spectroscopy
2012 Corporate Capabilities - Spectroscopy
2012 Corporate Capabilities - Spectroscopy
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
APPLICATION NOTES – DECEMBER 2011 Mass Spectrometry 93<br />
Simultaneous Qualitative and<br />
Quantitative Analysis of<br />
Buspirone and Its Metabolites<br />
with the Agilent 6550 iFunnel<br />
Q-TOF LC–MS System<br />
Yuqin Dai, Michael Flanagan, and Keith Waddell,<br />
Agilent Technologies, Inc.<br />
Timely and rapid assessment of metabolic stability, metabolite<br />
identification, and metabolite profiling is critical for accelerating<br />
lead optimization and enhancing the success rate of drug<br />
candidates entering into development. Traditionally, qualitative<br />
and quantitative analyses are often performed on different types<br />
of LC–MS instruments and in multiple runs. Te ability to obtain<br />
quantitation and identification (qual/quan) in a single analysis<br />
makes metabolic stability, metabolite identification, and metabolite<br />
profiling studies much more efficient. Tis note describes an<br />
integrated qual/quan workflow that is enabled by the sensitivity<br />
enhancement of iFunnel technology implementation on a quadrupole<br />
time-of-flight (Q-TOF) instrument. It demonstrates how Agilent<br />
6550 iFunnel Q-TOF permits high sensitivity, simultaneous<br />
determination of metabolic stability, metabolite identification, and<br />
metabolite profiling in an in vitro buspirone (1 μM) metabolism<br />
study in rat liver microsomes.<br />
An Integrated Qualitative/Quantitative Workflow<br />
Te qual/quan workflow starts with the LC–MS injection of a biological<br />
sample. Using the Agilent 6550 iFunnel Q-TOF LC–MS<br />
system, data acquisition includes a full MS scan followed by three<br />
data dependent auto MS-MS scans. Metabolite identification and<br />
structure elucidation are facilitated by MassHunter Metabolite ID<br />
software. Metabolic stability and metabolite profiling are established<br />
from the same set of data, which are processed in batch<br />
mode by MassHunter Quantitative Analysis software.<br />
Qual/Quan Analysis<br />
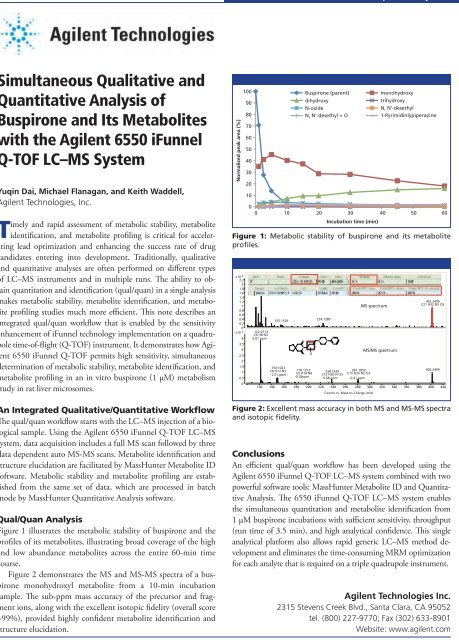
Figure 1 illustrates the metabolic stability of buspirone and the<br />
profiles of its metabolites, illustrating broad coverage of the high<br />
and low abundance metabolites across the entire 60-min time<br />
course.<br />
Figure 2 demonstrates the MS and MS-MS spectra of a buspirone<br />
monohydroxyl metabolite from a 10-min incubation<br />
sample. Te sub-ppm mass accuracy of the precursor and fragment<br />
ions, along with the excellent isotopic fidelity (overall score<br />
>99%), provided highly confident metabolite identification and<br />
structure elucidation.<br />
Normalized peak area (%)<br />
100 Buspirone (parent)<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
dihydroxy<br />
N-oxide<br />
N, N’-desethyl + O<br />
Incubation time (min)<br />
monohydroxy<br />
trihydroxy<br />
N, N’-desethyl<br />
1-Pyrimidinlypiperazine<br />
0<br />
0 10 20 30 40 50 60<br />
Figure 1: Metabolic stability of buspirone and its metabolite<br />
profiles.<br />
x10 5<br />
2.2 123.0918<br />
2<br />
1.8<br />
1.6<br />
1.4<br />
1.2<br />
1<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
0<br />
x10 4 122.0713<br />
C6 H8 N3<br />
4<br />
3.5<br />
3<br />
2.5<br />
2<br />
1.5<br />
1<br />
0.5<br />
0<br />
0.07 ppm<br />
155.1539<br />
O<br />
224.1280<br />
150.1023<br />
C8 H12 N3 178.1212 238.1439 281.1859<br />
-2.01 ppm<br />
C9 H14 N4 C13 H20 N O3 C15 H25 N2 O3<br />
-0.39ppm 0.68 ppm -0.41 ppm<br />
Counts vs. Mass-to-Charge (m/z)<br />
MS spectrum<br />
MS/MS spectrum<br />
402.2499<br />
C21 H32 N5 O3<br />
402.2499<br />
120 140 160 180 200 220 240 260 280 300 320 340 360 380 400 420<br />
Figure 2: Excellent mass accuracy in both MS and MS-MS spectra<br />
and isotopic fidelity.<br />
Conclusions<br />
An efficient qual/quan workflow has been developed using the<br />
Agilent 6550 iFunnel Q-TOF LC–MS system combined with two<br />
powerful software tools: MassHunter Metabolite ID and Quantitative<br />
Analysis. Te 6550 iFunnel Q-TOF LC–MS system enables<br />
the simultaneous quantitation and metabolite identification from<br />
1 μM buspirone incubations with sufficient sensitivity, throughput<br />
(run time of 3.5 min), and high analytical confidence. Tis single<br />
analytical platform also allows rapid generic LC–MS method development<br />
and eliminates the time-consuming MRM optimization<br />
for each analyte that is required on a triple quadrupole instrument.<br />
Agilent Technologies Inc.<br />
2315 Stevens Creek Blvd., Santa Clara, CA 95052<br />
tel. (800) 227-9770; Fax (302) 633-8901<br />
Website: www.agilent.com