Nucleic Acid Analysis with UV-vis and NMR - Spectroscopy
Nucleic Acid Analysis with UV-vis and NMR - Spectroscopy
Nucleic Acid Analysis with UV-vis and NMR - Spectroscopy
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
18 <strong>Spectroscopy</strong> 24(11) November 2009<br />
www.spectroscopyonline.com<br />
pressure measurements taken on-site,<br />
<strong>with</strong> the readings fed into a system<br />
that makes decisions on the fly. These<br />
complex topics deserve a more detailed<br />
exposition, which will appear in this<br />
column eventually. Until then, <strong>and</strong> to<br />
return to Earth orbit, interested readers<br />
might learn about the Wake Shield<br />
Facility (described at http://www.svec.<br />
uh.edu/wsfp.html), which takes advantage<br />
of the excellent vacuum available<br />
in the wake of the space shuttle.<br />
Earlier columns in “Mass Spectrometry<br />
Forum” covered general topics<br />
of vacuum systems (as well as our<br />
sometimes confusing uses of the terms<br />
vacuum <strong>and</strong> pressure), <strong>and</strong> the operation<br />
of high vacuum pumps (1,2). Here,<br />
to keep things simple, we will call any<br />
pressure below 1 atmosphere (760<br />
Torr) a vacuum. There are subsidiary<br />
terms of rough vacuum, low vacuum,<br />
high vacuum, <strong>and</strong> ultrahigh vacuum,<br />
<strong>with</strong> such terms corresponding, in<br />
order, to lower <strong>and</strong> lower pressures.<br />
The pressure in interstellar space, by<br />
the way, is about 10 -16 Torr, <strong>and</strong> the<br />
pressure <strong>with</strong>in interplanetary space<br />
higher (depending upon where you<br />
are). These are isotropic gas pressures,<br />
<strong>and</strong> the fact that the interstellar pressure<br />
is so low is one reason why radiation<br />
pressure can be used to exert a<br />
force upon solar sails.<br />
The focus of this column is the<br />
measurement of pressure in a mass<br />
spectrometer, located somewhere on<br />
the surface of planet Earth (+/- 5 km)<br />
(3). The continued growth <strong>and</strong> diversification<br />
of MS should refocus our<br />
attention on the attainment of vacuum<br />
<strong>and</strong> the accurate measurement of pressures.<br />
At the heart of MS is the ability<br />
to create ions, to move them around,<br />
to differentiate them by their mass-tocharge<br />
ratios, <strong>and</strong> to detect them. For<br />
years, <strong>and</strong> certainly through the dominant<br />
era for electron ionization (EI)<br />
<strong>and</strong> chemical ionization (CI) sources,<br />
we thought of the MS instrument<br />
as under high vacuum from source<br />
through to the detector. We also came<br />
to know the vacuum pumping system<br />
as a high-cost, high-maintenance part<br />
of the instrument. Certainly, pumping<br />
systems evolved from the crude apparatus<br />
first used by Aston in his<br />
Source<br />
Inlet<br />
Ionization gauge<br />
Analyzer<br />
Diffusion pump<br />
Thermocouple gauge<br />
Rough<br />
pump<br />
Detector<br />
vent<br />
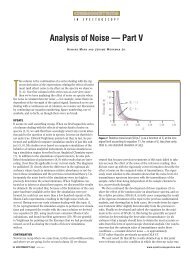
Figure 1: Simple diagram of placement of a thermocouple gauge (bottom) <strong>and</strong> an ionization<br />
gauge (top) on a generic mass spectrometer.<br />
Table I: Usual operational range for some common pressure gauges used<br />
in mass spectrometers.<br />
Device name<br />
Usual operational range (Torr)<br />
(760 Torr is 1 atmosphere of pressure)<br />
Mechanical gauge (various sorts) 1000 to 100<br />
Piezo or quartz sensor 760 to 1<br />
Capacitance manometer 1000 to 10 -3<br />
Thermocouple gauge 760 to 10 -3<br />
Ionization gauge 10 -4 to 10 -8<br />
parabola mass spectrographs, but the<br />
consistent general model was a highvacuum<br />
diffusion pump (or two) for<br />
the main system to achieve a high vacuum,<br />
<strong>with</strong> associated backing pumps<br />
(the rough pumps) that also could be<br />
plumbed into source interfaces for separators<br />
or direct insertion probes. Most<br />
pumps (including diffusion pumps<br />
<strong>and</strong> rotary vane backing pumps) transport<br />
gas molecules against a pressure<br />
gradient, so the ultimate exhaust for<br />
the backing pumps should be a hood<br />
vented to outside. The advent of reliable<br />
turbomolecular pumps did not<br />
shift the basic design of the pumping<br />
systems in our instruments, but added<br />
certain advantages of speed <strong>and</strong> pump<br />
placement. The basic goal still was to<br />
move the gas molecules from inside the<br />
system to outside of it.<br />
Within this general scheme, much of<br />
the plumbing intricacy of the vacuum<br />
pumping system was designed for the<br />
ionization source of the mass spectrometer,<br />
<strong>and</strong> the need to transport