Nucleic Acid Analysis with UV-vis and NMR - Spectroscopy
Nucleic Acid Analysis with UV-vis and NMR - Spectroscopy
Nucleic Acid Analysis with UV-vis and NMR - Spectroscopy
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
www.spectroscopyonline.com<br />
November 2009 <strong>Spectroscopy</strong> 23(11) 39<br />
indicated by the figure, the IC concentration<br />
has an obvious effect on ΔI RLS of<br />
the IC-CTMAB-DNA system. It is found<br />
that when the concentration of IC is in<br />
the range 2.0 X 10 -6 to 4..0 X 10 -6 mol/L,<br />
the ΔI RLS of the IC-CTMAB-DNA system<br />
reaches a maximum. So we select 3.0 X<br />
10 -6 mol/L IC for further research.<br />
Effect of the ionic strength<br />
The ionic strength of the medium<br />
has an effect on the interaction of IC,<br />
CTMAB, <strong>and</strong> DNA. As Figure 7 shows,<br />
the ΔI RLS of the IC-CTMAB-DNA system<br />
decreased slightly when NaCl was at<br />
a low concentration, while it decreased<br />
markedly <strong>with</strong> the increasing ionic<br />
strength. The reason could be that Na+<br />
shielded the phosphorus negative charge<br />
of DNA, which weakened the combination<br />
of DNA <strong>and</strong> CTMAB, so the ΔI RLS<br />
decreased, despite the strong dependence<br />
of the enhanced light scattering of IC by<br />
DNA upon ionic strength ranges from 0<br />
to 0.05.<br />
Reaction time <strong>and</strong> stability<br />
The binding reaction of DNA <strong>and</strong> IC<br />
<strong>with</strong> CTMAB occurs rapidly at room<br />
temperature after less than 2 min, <strong>and</strong><br />
the ΔI RLS remains a constant for about<br />
4 h. It shows that the reaction does not<br />
require any crucial timing <strong>and</strong> has good<br />
stability.<br />
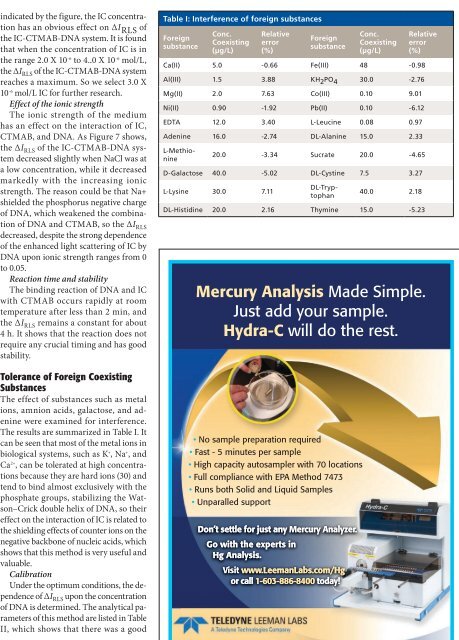
Table I: Interference of foreign substances<br />
Foreign<br />
substance<br />
Conc.<br />
Coexisting<br />
(µg/L)<br />
Relative<br />
error<br />
(%)<br />
Foreign<br />
substance<br />
Conc.<br />
Coexisting<br />
(µg/L)<br />
Relative<br />
error<br />
(%)<br />
Ca(II) 5.0 -0.66 Fe(III) 48 -0.98<br />
Al(III) 1.5 3.88 KH 2 PO 4 30.0 -2.76<br />
Mg(II) 2.0 7.63 Co(III) 0.10 9.01<br />
Ni(II) 0.90 -1.92 Pb(II) 0.10 -6.12<br />
EDTA 12.0 3.40 L-Leucine 0.08 0.97<br />
Adenine 16.0 -2.74 DL-Alanine 15.0 2.33<br />
20.0 -3.34 Sucrate 20.0 -4.65<br />
D-Galactose 40.0 -5.02 DL-Cystine 7.5 3.27<br />
L-Lysine 30.0 7.11<br />
L-Methionine<br />
DL-Tryptophan<br />
40.0 2.18<br />
DL-Histidine 20.0 2.16 Thymine 15.0 -5.23<br />
Tolerance of Foreign Coexisting<br />
Substances<br />
The effect of substances such as metal<br />
ions, amnion acids, galactose, <strong>and</strong> adenine<br />
were examined for interference.<br />
The results are summarized in Table I. It<br />
can be seen that most of the metal ions in<br />
biological systems, such as K + , Na + , <strong>and</strong><br />
Ca 2+ , can be tolerated at high concentrations<br />
because they are hard ions (30) <strong>and</strong><br />
tend to bind almost exclusively <strong>with</strong> the<br />
phosphate groups, stabilizing the Watson–Crick<br />
double helix of DNA, so their<br />
effect on the interaction of IC is related to<br />
the shielding effects of counter ions on the<br />
negative backbone of nucleic acids, which<br />
shows that this method is very useful <strong>and</strong><br />
valuable.<br />
Calibration<br />
Under the optimum conditions, the dependence<br />
of ΔI RLS upon the concentration<br />
of DNA is determined. The analytical parameters<br />
of this method are listed in Table<br />
II, which shows that there was a good