Nucleic Acid Analysis with UV-vis and NMR - Spectroscopy
Nucleic Acid Analysis with UV-vis and NMR - Spectroscopy
Nucleic Acid Analysis with UV-vis and NMR - Spectroscopy
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
20 <strong>Spectroscopy</strong> 24(11) November 2009 www.spectroscopyonline.com<br />
mass analyzer may be the cause. The<br />
author of this quote addresses the need<br />
for accurate pressure measurement in<br />
a vacuum chamber used for materials<br />
processing, but the lesson is valuable.<br />
For the mass spectrometer, scattering<br />
collisions reduce ion transmission,<br />
<strong>and</strong> reduce instrument sensitivity. A<br />
“small” change in pressure can lead<br />
to an amplified degradation in performance.<br />
Pumps “burp” for various<br />
reasons, <strong>and</strong> the short pressure surge<br />
takes time to dissipate. Erratic instrument<br />
performance may be your only<br />
clue if you are not carefully monitoring<br />
the pressure. The author may be the<br />
only mass spectrometrist in the world<br />
who would become concerned <strong>with</strong> a<br />
shift in measured base pressure from<br />
2 X 10 -6 to 3 X 10 -6 Torr, but now you<br />
know why.<br />
Performance of the vacuum pumping<br />
system of a mass spectrometer<br />
is monitored through measurement<br />
of the pressure at various points in<br />
the system. These measured pressures<br />
may or may not be logged into<br />
the automated control system of the<br />
mass spectrometer. In process vacuum<br />
chambers, system pressures certainly<br />
are recorded so that the conditions of<br />
materials processing are known precisely.<br />
But in analytical mass spectrometers,<br />
pressure measurement is often<br />
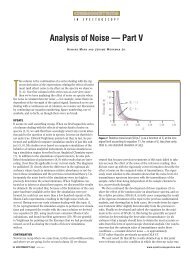
manually monitored. Consider Figure<br />
1, which is a schematic of a diffusion<br />
pump attached to the main vacuum<br />
chamber, supported by the backing<br />
pump (also known as the rough pump),<br />
<strong>with</strong> the system exhaust routed to a<br />
hood. Pressures <strong>with</strong>in the backing<br />
pump lines are monitored (using the<br />
thermocouple gauge) as an indicator of<br />
the total gas load on the system, <strong>and</strong> to<br />
ensure that the main diffusion pump is<br />
properly supported. The main chamber<br />
pressure is monitored <strong>with</strong> the<br />
ionization gauge. The diffusion pump<br />
can operate for a short time at higher<br />
backing pressures, but prolonged operation<br />
leads inevitably to a rise in main<br />
system pressure <strong>and</strong> a degradation of<br />
the pumping fluid. In a simple EI mass<br />
spectrometer, monitoring the two pressures<br />
(the backing pump line thermocouple<br />
gauge <strong>and</strong> the main chamber<br />
ionization gauge) usually is sufficient<br />
for reassurance that the instrument<br />
was properly pumped.<br />
Details of how each measurement<br />
device works are explored in specialized<br />
texts (5–8) <strong>and</strong> commercial manufacturer’s<br />
applications notes, reflecting<br />
the broad application of vacuum<br />
science outside of MS. We concentrate<br />
here on the thermocouple gauge <strong>and</strong><br />
the ionization gauge, because these are<br />
the most common devices found on<br />
mass spectrometers, usually configured<br />
approximately as shown in Figure<br />
1. Table I shows the operational pressure<br />
range for the thermocouple gauge<br />
<strong>and</strong> for the ionization gauge, <strong>and</strong> a few<br />
other devices included for comparison.<br />
Performance of<br />
the vacuum<br />
pumping<br />
system of a mass<br />
spectrometer is<br />
monitored through<br />
measurement of<br />
the pressure at<br />
various points in<br />
the system.<br />
Note that in addition to monitoring<br />
the pressure in the backing lines, thermocouple<br />
gauges also can be used to<br />
monitor vacuum pressures in sample<br />
introduction interfaces. Note that for<br />
sources that operate at atmospheric<br />
pressure, the ambient pressure usually<br />
is not measured. We emphasize in this<br />
column three basic issues relevant to<br />
the vacuum pumping system in a mass<br />
spectrometer. First, the vacuum gauge<br />
produces an electrical output that can<br />
be related to pressure through a calibration,<br />
<strong>and</strong> not all residual gas mixtures<br />
follow the same calibration curve.<br />
Second, the pressure measured is the<br />
pressure at the gauge, not necessarily in<br />
the system, <strong>and</strong> so we have to consider<br />
conductance. Third <strong>and</strong> finally, proper<br />
vacuum-pumping system care<br />
maintains instrument performance.<br />
Calibration: Like any transducer,<br />
a vacuum gauge must be calibrated.<br />
In MS, that calibration entails some<br />
special issues. The composition of a gas<br />
mixture at Earth atmospheric pressure<br />
contains an expected mix of nitrogen,<br />
oxygen, water, carbon dioxide, argon,<br />
<strong>and</strong> some trace components. Does this<br />
mixture change in its relative composition<br />
as a pump lowers the pressure in<br />
a vacuum chamber? It most certainly<br />
does, because various pumps may act<br />
to pump one gas component more effectively<br />
than another. Thus the composition<br />
of that starting gas mixture<br />
at 10 -3 Torr will be slightly different<br />
from the composition at atmosphere,<br />
even more different at 10 -6 Torr, <strong>and</strong><br />
vastly different at 10 -9 Torr. For the<br />
pressure-measuring devices, a calibration<br />
should be completed so that the<br />
electrical output can be related to the<br />
actual pressure, <strong>and</strong> the calibration<br />
must factor in the composition of the<br />
gas. Any device reacts to different gases<br />
<strong>with</strong> a different sensitivity, <strong>and</strong> thus<br />
exhibits a different calibration curve.<br />
A thermocouple gauge that measures<br />
1 Torr of pressure when the composition<br />
is residual atmospheric gases will<br />
be slightly different in response from<br />
a thermocouple gauge reading 1 Torr<br />
of methane in a CI source. Many commercial<br />
companies offer calibration<br />
services for vacuum gauges. In MS,<br />
traceability to a NIST or other national<br />
st<strong>and</strong>ard is not usually necessary. NIST<br />
calibration is described in detail in<br />
publications available on the web (9,10).<br />
Conductance: The connection of<br />
a vacuum gauge to the mass spectrometer<br />
deserves some discussion.<br />
Consider the two situations depicted<br />
in Figure 1 for a thermocouple gauge<br />
<strong>and</strong> for an ionization gauge. The thermocouple<br />
gauge is connected directly<br />
into the vacuum line, <strong>and</strong> many gauges<br />
are built into threaded assemblies for<br />
such a purpose. We can be assured<br />
that the pressure measured by the<br />
thermocouple gauge is the pressure in<br />
the line because of this direct connection.<br />
On the other h<strong>and</strong>, consider the<br />
connection of an ionization gauge to<br />
the main vacuum chamber of a mass<br />
spectrometer. Sometimes the glass-en-