Nucleic Acid Analysis with UV-vis and NMR - Spectroscopy
Nucleic Acid Analysis with UV-vis and NMR - Spectroscopy
Nucleic Acid Analysis with UV-vis and NMR - Spectroscopy
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
24 <strong>Spectroscopy</strong> 24(11) November 2009 www.spectroscopyonline.com<br />
• “The agency must place greater<br />
emphasis on significant risks <strong>and</strong> violations,<br />
<strong>and</strong> use meaningful penalties<br />
to send a strong message to discourage<br />
future offenses.”<br />
The strong message coming through<br />
loud <strong>and</strong> clear is be compliant <strong>and</strong><br />
remain compliant <strong>with</strong> the regulations<br />
— or else. You’ll now see that the tiger,<br />
far from being ready for the taxidermist,<br />
is starting to sharpen its teeth.<br />
Not only does this apply to the<br />
here-<strong>and</strong>-now but also, as noted in the<br />
final bullet point, the commissioner<br />
wants to discourage future offences.<br />
So why change the FDA approach to<br />
enforcement? One of the rationales for<br />
this was mentioned in the speech as<br />
the pathways for enforcement action<br />
can be too long <strong>and</strong> arduous when the<br />
public’s health is in jeopardy. So from<br />
this <strong>and</strong> the FDA, we must infer that<br />
the FDA will be increasing inspections<br />
while tightening up <strong>and</strong> speeding up<br />
enforcement actions.<br />
Thus. it was not surprising that the<br />
FDA announced in the Federal Register<br />
of August 11 (4) the trial of a new<br />
program entitled “Review of Post-Inspection<br />
Responses.” The purpose of<br />
this program is to facilitate the timely<br />
issuance of warning letters that started<br />
on September 15, 2009 <strong>and</strong> will run for<br />
18 months (the tiger has just come back<br />
from the dentist <strong>and</strong> is feeling hungry<br />
— are you getting the message?).<br />
Issues <strong>with</strong> the Current<br />
Inspection Process<br />
Before we look in detail at the inspection<br />
goodies coming your way, I would<br />
just like to step back <strong>and</strong> review what<br />
happened before implementation of<br />
this new program. FDA inspections<br />
come in three flavors <strong>and</strong> will continue<br />
to do so:<br />
• Facility inspection: routine inspection<br />
of the facility <strong>and</strong> the six areas<br />
quality — however, we will just focus<br />
on the quality system <strong>and</strong> the laboratory<br />
in this column.<br />
• Preapproval inspection (PAI): inspection<br />
of the manufacturing facility<br />
for a new drug or a generic drug before<br />
licensing.<br />
• For cause inspection: either there<br />
is a public health issue that must be<br />
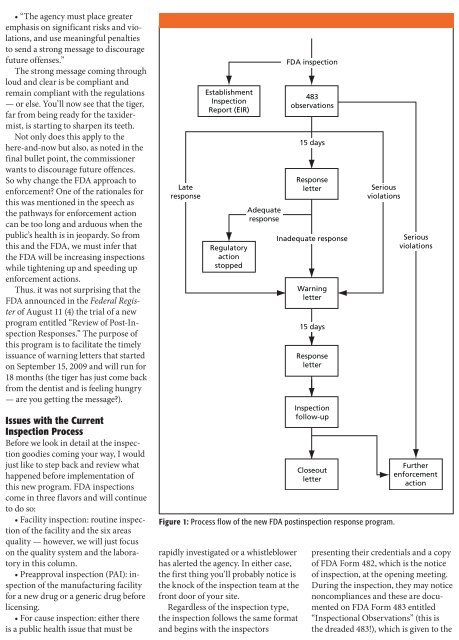
Late<br />
response<br />
Establishment<br />
Inspection<br />
Report (EIR)<br />
Regulatory<br />
action<br />
stopped<br />
Adequate<br />
response<br />
rapidly investigated or a whistleblower<br />
has alerted the agency. In either case,<br />
the first thing you’ll probably notice is<br />
the knock of the inspection team at the<br />
front door of your site.<br />
Regardless of the inspection type,<br />
the inspection follows the same format<br />
<strong>and</strong> begins <strong>with</strong> the inspectors<br />
FDA inspection<br />
483<br />
observations<br />
15 days<br />
Response<br />
letter<br />
Inadequate response<br />
Warning<br />
letter<br />
15 days<br />
Response<br />
letter<br />
Inspection<br />
follow-up<br />
Closeout<br />
letter<br />
Serious<br />
violations<br />
Figure 1: Process flow of the new FDA postinspection response program.<br />
Serious<br />
violations<br />
Further<br />
enforcement<br />
action<br />
presenting their credentials <strong>and</strong> a copy<br />
of FDA Form 482, which is the notice<br />
of inspection, at the opening meeting.<br />
During the inspection, they may notice<br />
noncompliances <strong>and</strong> these are documented<br />
on FDA Form 483 entitled<br />
“Inspectional Observations” (this is<br />
the dreaded 483!), which is given to the