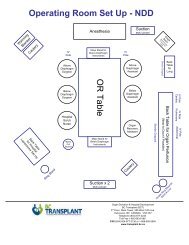
4. Clinical Guidelines for Liver Transplantation (PDF) - British ...
4. Clinical Guidelines for Liver Transplantation (PDF) - British ...
4. Clinical Guidelines for Liver Transplantation (PDF) - British ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Guidebook <strong>for</strong> the Solid Organ Transplant Programme Chapter 4<br />
<strong>4.</strong>3.2 EARLY COMPLICATIONS CONT.<br />
2. Mycophenolate Mofetil (CellCept )<br />
In the maintenance phase, Mycophenolate Mofetil (MMF) use is restricted to the following<br />
indications:<br />
a. Renal dysfunction: Under these circumstances, in order to allow the use of low-dose<br />
calcineurin inhibitors or, in some cases, no calcineurin inhibitor. Mycophenolate<br />
Mofetil is used.<br />
b. Graft rejection: In circumstances in which graft rejection occurs despite the use of<br />
Tacrolimus at therapeutic levels, Mycophenolate Mofetil will be used. Once graft<br />
function has stabilized, Mycophenolate Mofetil use may be discontinued after three<br />
to six months.<br />
c. Calcineurin neurotoxicity: In circumstances where neurotoxicity has occurred to a<br />
calcineurin inhibiting agent, Mycophenolate Mofetil use will be indicated on an<br />
indefinite basis.<br />
d. Mycophenolate Mofetil use as a steroid-sparing agent: There may be circumstances<br />
in which patient’s should be weaned off maintenance Prednisone, i.e. osteoporosis,<br />
osteopenia, diabetic mellitus, etc. and <strong>for</strong> which patients are either intolerant of<br />
Azathioprine or the continued use of Azathioprine is inadequate to maintain a<br />
rejection-free allograft. Under these circumstances, Mycophenolate Mofetil may<br />
have to be used on an indefinite basis.<br />
The goal is <strong>for</strong> all patients that are clinically stable and have reached one year on initial<br />
immunosuppression with Mycophenolate to switch to Azathioprine 1 mg/kg/day PO except<br />
patients with the following:<br />
<br />
<br />
<br />
Calcineurin inhibitor nephrotoxicity or neurotoxicity despite therapeutic<br />
calcineurin inhibitor concentrations<br />
Calcineurin inhibitor hypersensitivity reactions or microangiopathy<br />
Multiple rejection episodes (≥ 2 rejections within the first year post transplant),<br />
despite adequate maintenance immunosuppression, including the inability to<br />
discontinue steroids<br />
3. Sirolimus (Rapamune ® )<br />
The use of Sirolimus by the <strong>Liver</strong> Transplant Program is on a case by case individualized<br />
basis. Due to an increased risk of hepatic artery thrombosis (HAT) in de novo transplant<br />
recipients, Sirolimus should not be used within the first 3 months of transplantation. The<br />
risk/benefit ratio of Sirolimus use in any liver transplant recipients must be discussed with<br />
the liver transplant team and the patient. Sirolimus has a long half-life (around 72 hours)<br />
and takes about 7 to 10 days to reach therapeutic level. There<strong>for</strong>e it should be used with<br />
another agent until therapeutic level is reached. (See Appendix E: Target Sirolimus<br />
Therapeutic Blood Concentrations)<br />
Chapter 4 – <strong>Clinical</strong> <strong>Guidelines</strong> <strong>for</strong> <strong>Liver</strong> <strong>Transplantation</strong> – July, 2010 Page 14<br />
See Page 1 <strong>for</strong> disclaimer