4. Clinical Guidelines for Liver Transplantation (PDF) - British ...
4. Clinical Guidelines for Liver Transplantation (PDF) - British ...
4. Clinical Guidelines for Liver Transplantation (PDF) - British ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Guidebook <strong>for</strong> the Solid Organ Transplant Programme Chapter 4<br />
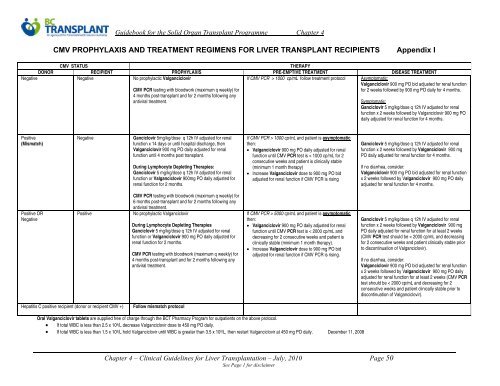
CMV PROPHYLAXIS AND TREATMENT REGIMENS FOR LIVER TRANSPLANT RECIPIENTS Appendix I<br />
CMV STATUS<br />
THERAPY<br />
DONOR RECIPIENT PROPHYLAXIS PRE-EMPTIVE TREATMENT DISEASE TREATMENT<br />
Negative Negative No prophylactic Valganciclovir<br />
If CMV PCR > 1000 cp/mL follow treatment protocol<br />
CMV PCR testing with bloodwork (maximum q weekly) <strong>for</strong><br />
4 months post-transplant and <strong>for</strong> 2 months following any<br />
antiviral treatment.<br />
Asymptomatic:<br />
Valganciclovir 900 mg PO bid adjusted <strong>for</strong> renal function<br />
<strong>for</strong> 2 weeks followed by 900 mg PO daily <strong>for</strong> 4 months.<br />
Symptomatic:<br />
Ganciclovir 5 mg/kg/dose q 12h IV adjusted <strong>for</strong> renal<br />
function x 2 weeks followed by Valganciclovir 900 mg PO<br />
daily adjusted <strong>for</strong> renal function <strong>for</strong> 4 months.<br />
Positive<br />
(Mismatch)<br />
Negative<br />
Ganciclovir 5mg/kg/dose q 12h IV adjusted <strong>for</strong> renal<br />
function x 14 days or until hospital discharge, then<br />
Valganciclovir 900 mg PO daily adjusted <strong>for</strong> renal<br />
function until 4 months post transplant.<br />
During Lymphocyte Depleting Therapies:<br />
Ganciclovir 5 mg/kg/dose q 12h IV adjusted <strong>for</strong> renal<br />
function or Valganciclovir 900mg PO daily adjusted <strong>for</strong><br />
renal function <strong>for</strong> 2 months.<br />
If CMV PCR > 1000 cp/mL and patient is asymptomatic<br />
then:<br />
Valganciclovir 900 mg PO daily adjusted <strong>for</strong> renal<br />
function until CMV PCR test is < 1000 cp/mL <strong>for</strong> 2<br />
consecutive weeks and patient is clinically stable<br />
(minimum 1 month therapy)<br />
Increase Valganciclovir dose to 900 mg PO bid<br />
adjusted <strong>for</strong> renal function if CMV PCR is rising<br />
Ganciclovir 5 mg/kg/dose q 12h IV adjusted <strong>for</strong> renal<br />
function x 2 weeks followed by Valganciclovir 900 mg<br />
PO daily adjusted <strong>for</strong> renal function <strong>for</strong> 4 months.<br />
If no diarrhea, consider:<br />
Valganciclovir 900 mg PO bid adjusted <strong>for</strong> renal function<br />
x 2 weeks followed by Valganciclovir 900 mg PO daily<br />
adjusted <strong>for</strong> renal function <strong>for</strong> 4 months.<br />
Positive OR<br />
Negative<br />
Positive<br />
CMV PCR testing with bloodwork (maximum q weekly) <strong>for</strong><br />
6 months post-transplant and <strong>for</strong> 2 months following any<br />
antiviral treatment.<br />
No prophylactic Valganciclovir<br />
During Lymphocyte Depleting Therapies<br />
Ganciclovir 5 mg/kg/dose q 12h IV adjusted <strong>for</strong> renal<br />
function or Valganciclovir 900 mg PO daily adjusted <strong>for</strong><br />
renal function <strong>for</strong> 2 months.<br />
CMV PCR testing with bloodwork (maximum q weekly) <strong>for</strong><br />
4 months post-transplant and <strong>for</strong> 2 months following any<br />
antiviral treatment.<br />
If CMV PCR > 5000 cp/mL and patient is asymptomatic<br />
then:<br />
Valganciclovir 900 mg PO daily adjusted <strong>for</strong> renal<br />
function until CMV PCR test is < 2000 cp/mL and<br />
decreasing <strong>for</strong> 2 consecutive weeks and patient is<br />
clinically stable (minimum 1 month therapy).<br />
Increase Valganciclovir dose to 900 mg PO bid<br />
adjusted <strong>for</strong> renal function if CMV PCR is rising.<br />
Ganciclovir 5 mg/kg/dose q 12h IV adjusted <strong>for</strong> renal<br />
function x 2 weeks followed by Valganciclovir 900 mg<br />
PO daily adjusted <strong>for</strong> renal function <strong>for</strong> at least 2 weeks<br />
(CMV PCR test should be < 2000 cp/mL and decreasing<br />
<strong>for</strong> 2 consecutive weeks and patient clinically stable prior<br />
to discontinuation of Valganciclovir).<br />
If no diarrhea, consider:<br />
Valganciclovir 900 mg PO bid adjusted <strong>for</strong> renal function<br />
x 2 weeks followed by Valganciclovir 900 mg PO daily<br />
adjusted <strong>for</strong> renal function <strong>for</strong> at least 2 weeks (CMV PCR<br />
test should be < 2000 cp/mL and decreasing <strong>for</strong> 2<br />
consecutive weeks and patient clinically stable prior to<br />
discontinuation of Valganciclovir).<br />
Hepatitis C positive recipient (donor or recipient CMV +) Follow mismatch protocol<br />
Oral Valganciclovir tablets are supplied free of charge through the BCT Pharmacy Program <strong>for</strong> outpatients on the above protocol.<br />
If total WBC is less than 2.5 x 10 9 /L decrease Valganciclovir dose to 450 mg PO daily.<br />
If total WBC is less than 1.5 x 10 9 /L hold Valganciclovir until WBC is greater than 3.5 x 10 9 /L, then restart Valganciclovir at 450 mg PO daily. December 11, 2008<br />
Chapter 4 – <strong>Clinical</strong> <strong>Guidelines</strong> <strong>for</strong> <strong>Liver</strong> <strong>Transplantation</strong> – July, 2010 Page 50<br />
See Page 1 <strong>for</strong> disclaimer