Atomic Layer Deposition at UCSB
Atomic Layer Deposition at UCSB
Atomic Layer Deposition at UCSB
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Atomic</strong> <strong>Layer</strong> <strong>Deposition</strong> @ <strong>UCSB</strong><br />
• Oxford FlexAL ALD tool<br />
• Load locked, turbo pumped unit<br />
• System pressures below 240 mTorr<br />
• Substr<strong>at</strong>e temps up to 500C<br />
• Configured for 6 metal-organic precursors<br />
• 8 gas lines configured for 6 reactive gases<br />
plus Ar (bubbling/purge) and w<strong>at</strong>er<br />
• Reactive gases: O2, N2, H2, NH3, and O3<br />
• In-situ monitoring of ALD process with<br />
Woollam Spectroscopic Ellipsometer<br />
• ICP Remote Plasma source (up to 600W)<br />
• Ozone Gener<strong>at</strong>or and Controller recently<br />
added
Films Grown/ Precursors Used @ <strong>UCSB</strong><br />
Standard “already developed” Processes<br />
• Al2O3 growth<br />
• Thermal: TMA + H2O<br />
• Plasma: TMA + O2<br />
• HfO2 growth<br />
• Thermal: TEMAH + H2O<br />
• Plasma: TEMAH + O2<br />
• ZrO2 growth<br />
• Plasma: ZrEMA + O2<br />
• SiO2 growth<br />
• Plasma: 3DMAS + O2<br />
• SiNx growth<br />
• Plasma: 3DMAS + H2/N2<br />
• TiO2 growth<br />
• Thermal: TTIP + H2O<br />
• TiN growth<br />
• Plasma: TDMAT + H2/N2<br />
New “under development” Processes<br />
• SiO2 growth<br />
• Thermal: BDEAS + O3<br />
• TiN growth<br />
• Thermal: TDMAT + NH3<br />
• Ru metal film growth<br />
• Thermal: Ru(Cp)2 + O2<br />
• Thermal: Ru(Cp)2 + O3
Ozone for “Active” Thermal Oxid<strong>at</strong>ion<br />
InUSA MiniODS Ozone Source + Sci-L Controller<br />
• Closed loop feedback control of O3 concentr<strong>at</strong>ion<br />
using built-in O3 monitor.<br />
• O2 gas flows up to 1000 sccm, O3 concentr<strong>at</strong>ion up<br />
to 22 wt%.<br />
• When gener<strong>at</strong>or is on, O3 is always flowing => need a<br />
divert line and a process line (built-in with source)<br />
• Divert line must be pumped since the gener<strong>at</strong>or<br />
runs @ 30 psig (maintained by BPR) and the ALD<br />
unit is low pressure.<br />
• Process line connects to reactive gas pod of the<br />
ALD unit => can use standard ALD valves already<br />
in place (small modific<strong>at</strong>ion of the FlexAL control<br />
software was required)<br />
• We use a small script to control the ozone source<br />
(gas flow, O3 concentr<strong>at</strong>ion, On/Off) directly from<br />
the desktop of the ALD control PC<br />
OZONE SOURCE<br />
divert<br />
gener<strong>at</strong>or<br />
bpr<br />
process<br />
<strong>at</strong>mosphere<br />
ALD unit<br />
Gas Pod
Applic<strong>at</strong>ion: Conductive TiN Film Growth<br />
<strong>Deposition</strong> conditions<br />
• Substr<strong>at</strong>e temp = 300C,<br />
• TDMAT cycle: 1sec @ 15mT , Ar draw @ 50sccm;<br />
Ar purge 4s @ 15mT; source @ 60C<br />
• H2/N2 plasma dosing: flow, pressure, ICP power,<br />
time are variable<br />
Annealing conditions (resistivity stability tests)<br />
• 60 min @ 400C in Forming Gas<br />
1.2<br />
1400<br />
GR (A/cycle)<br />
1<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
0<br />
0 10 20 30 40 50<br />
H2/N2 Plasma Time (s)<br />
15mT, 30/10<br />
H2/N2 400W<br />
10o’sK uOhm-cm)<br />
• Resistivity less sensitive to plasma time @<br />
lower ICP Power and/or H2-N2 r<strong>at</strong>io
Capacitance ( µF / cm 2 )<br />
Applic<strong>at</strong>ion: MIS devices on III-V<br />
semiconductors<br />
• Why Al2O3 To improve III-V films MIS? Grown EOT InGaAs of insul<strong>at</strong>or, on InGaAs has higher need electron higher dielectric velocity and constant lower m<strong>at</strong>erial Eg => faster => HfO2! electronics and lower power<br />
demand.<br />
• <strong>Deposition</strong> Found • In-situ th<strong>at</strong> As Conditions HfO2 decapping would using not FlexAL deposit 50 cycles ALD cleanly TMA/H2O on InGaAs @ 300C => => use 5nm interfacial Al2O3 layer of Al2O3<br />
Scaling • TMA(RT) requires • cycle: thin 20ms ( perfect Ar purge for ALD 7s @ 15mT; pump 2s<br />
• <strong>Deposition</strong> Condition<br />
Grow amorphous<br />
uses 10 cycles<br />
As-cap<br />
TMA/H2O<br />
on InGaAs<br />
then<br />
<strong>at</strong> the<br />
40<br />
end<br />
cycles<br />
of<br />
TEMAH/H2O<br />
MBE growth<br />
@<br />
=><br />
300C<br />
surface is protected from<br />
Major H2O cycle: issue 100ms; – maintain Ar purge low 7s @ 15mTorr; st<strong>at</strong>e density pump (Dit) 7s @ growth interface<br />
• TMA(RT)/TEMAH(70C)<br />
environment (ideal<br />
cycle:<br />
surface<br />
500ms<br />
=><br />
@<br />
minimal<br />
200mT<br />
Dit?).<br />
, Ar draw @ 100sccm; Ar purge 7s @ 15mT; pump 2s<br />
• InGaAs Cleaning • H2O MIS cycle: Tre<strong>at</strong>ment • As- @ 500ms; <strong>UCSB</strong> cap removed prior utilizes Ar purge to growth a by g<strong>at</strong>e 7s exposure @ last using 15mTorr; (dummy to 10 low cycles pump g<strong>at</strong>e) power of 7s TMA/H* process.<br />
plasma plasma (2W) @ as 300C AsH3. Cycle (2W H* plasma for<br />
• interface TMA cycle: 5s)/(SE is exposed 40ms measurement @ to 200mT air and , Ar for processing draw 4s) until @ 100sccm; chemicals interface Ar reached. prior purge to 5s ALD @ growth 15mT; pump => high 2s Dit<br />
• Same cleaning tre<strong>at</strong>ment prior to growth using 5 cycles of TMA/H* plasma @ 300C<br />
• H* plasma • Immedi<strong>at</strong>e cycle: 2s oxide @ 100W growth ICP/20mT, on clean 50sccm InGaAs H2; interface Ar purge (interface 5s @ 15mTorr; is never pump exposed 2s to air!)<br />
• Initial results promising<br />
1.6<br />
1.4<br />
1.2<br />
1<br />
InAs<br />
0.8<br />
0.6 S/D<br />
No H 2<br />
/ TMA Cycles<br />
“False<br />
inversion”<br />
“Stretch<br />
out”<br />
0.4<br />
100 Hz<br />
1 kHz<br />
10 kHz<br />
0.2<br />
100 kHz<br />
InGaAs<br />
InAlAs Back Barrier<br />
1 MHz<br />
0<br />
-3 -2 -1 0 1 2 3 -3 -2 -1 0 1 2 3<br />
Voltage (V)<br />
Voltage (V)<br />
H 2<br />
Nickel Al2O3/InGaAs G<strong>at</strong>e<br />
Metal<br />
1 00 nm L g<br />
1 0 nm InGaAs Channel<br />
STEM FET cross section, color-coded by<br />
chemistry<br />
+ TM A Cycles<br />
As-cap<br />
Low- damage process<br />
Thermal<br />
HfO2/Al2O3/InGaAs<br />
g<strong>at</strong>e metal<br />
No/Low- damage plasma<br />
• TMA/H* cycling improves interface<br />
significantly:<br />
• False inversion in ‘depletion’<br />
suppressed<br />
week)<br />
• Transition from depletion to<br />
accumul<strong>at</strong>ion rapid is sharper learning<br />
Rapid turn- around (~2<br />
ALD Oxide Process<br />
Al 2 O 3 /HfO 2 Bilayer<br />
Highly Conformal!
SiOx <strong>Deposition</strong> R<strong>at</strong>e (A/cycle)<br />
0.90<br />
0.80<br />
0.70<br />
0.60<br />
0.50<br />
0.40<br />
0.30<br />
0.20<br />
BDEAS@50mTorr/3s<br />
O3@200mTorr<br />
O3@50mTorr<br />
0.10<br />
0 5 10 15 20<br />
O3 Dose (secs)<br />
Applic<strong>at</strong>ion: SiOx ALD Growth using<br />
“SAM.24” and Ozone<br />
• Standard S<strong>at</strong>ur<strong>at</strong>ion SiOx Film aminosilane growth Growth using R<strong>at</strong>e precursor aminosilane strongly for dependent SiOx precursors film on growth requires Ozone in Pressure ALD an ‘active’ => TDMAS (or form adsorption of oxygen flux)! (CH3) 2 N N(CH3) 2<br />
• Contains suggests 2 options three th<strong>at</strong> – plasma O3 dimethyl dissoci<strong>at</strong>ion O* source amino or is groups balanced thermal O3 by source desorption from molecularly adsorbed st<strong>at</strong>e. Si<br />
• Removal Here BDEAS we adsorption investig<strong>at</strong>e of first 2 amino is BDEAS pressure groups + O3 independent during thermal dissoci<strong>at</strong>ive ALD => (preliminary irreversible chemisorption and study) complete are<br />
H N(CH3)<br />
thermodynamically favorable.<br />
2<br />
• <strong>Deposition</strong> Conditions using BDEAS/O3 cycling <strong>at</strong> 300C<br />
• Due BDEAS(50C) to strain, cycle: removal 100% of vapor final amino flow @ group 50 or is 200 not mTorr; favorable Ar purge => issues @ 50sccm/2s; with C/N pump 3s<br />
incorpor<strong>at</strong>ion in film<br />
• O3 cycle: 250sccm O2 flow/19 wt% O3 conc @ 50 or 200 mTorr; O3(g) Ar purge @ 50sccm/2s; pump 3s<br />
• “New” aminosilane precursor (Air Liquide – SAM.24) => BDEAS<br />
(C2H5) 2 N N(C2H5) 2<br />
• Contains only two diethyl amino groups which are easily removed during<br />
dissoci<strong>at</strong>ive chemisorption<br />
O3(a)<br />
Si<br />
• Cleaner SiOx films?<br />
O2(g)+O(a)<br />
H H<br />
SiOx <strong>Deposition</strong> R<strong>at</strong>e @ S<strong>at</strong>ur<strong>at</strong>ion<br />
(A/cycle)<br />
0.90<br />
0.85<br />
0.80<br />
0.75<br />
0.70<br />
0.65<br />
0.60<br />
0.55<br />
0.50<br />
0.45<br />
BDEAS dose = 3s,<br />
(O3@200mTorr/10s)<br />
O3 dose = 10s,<br />
(BDEAS@50mTorr/3s)<br />
0.40<br />
50 100 150 200<br />
Gas Pressure (mTorr)
A few more applic<strong>at</strong>ions…<br />
High performance N-Polar GaN-HEMTs using<br />
5nm Al2O3 G<strong>at</strong>e Oxide– Denninghoff et. al.<br />
Dit reduction of ALD-Al2O3 on Ga-faced GaN using<br />
w<strong>at</strong>er pre-s<strong>at</strong>ur<strong>at</strong>ion of surface – X Liu et. al.<br />
No H2O<br />
H2O tre<strong>at</strong>ed<br />
Near Midgap Dit St<strong>at</strong>es dropped by an order of magnitude (5e11cm -2 eV -1<br />
to 4e10 cm -2 eV -1 ) when pre-tre<strong>at</strong>ing surface with H2O before ALD growth<br />
1.4 A/mm, Good Turn-off, 270GHz fmax.<br />
ALD-Al2O3 for SiO2 membrane pore size shrinking and ion-current leakage suppression for CMOScomp<strong>at</strong>ible<br />
Biosensing devices – A. Uddin, etl al.<br />
Pores<br />
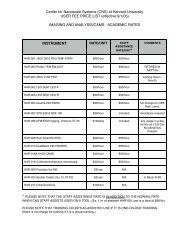
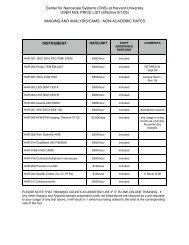
EXPERIMENTAL SETUP<br />
I-V Characteristics of blank SiOx membrane<br />
before and after ALD co<strong>at</strong>ing.