Topical tacrolimus in atopic dermatitis: Effects of ... - Helda - Helsinki.fi
Topical tacrolimus in atopic dermatitis: Effects of ... - Helda - Helsinki.fi
Topical tacrolimus in atopic dermatitis: Effects of ... - Helda - Helsinki.fi
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
SUBJECTS AND METHODS<br />
Study designs<br />
All studies were carried out <strong>in</strong> Sk<strong>in</strong> and Allergy Hospital, Hels<strong>in</strong>ki University Central<br />
Hospital, Hels<strong>in</strong>ki, F<strong>in</strong>land, and were approved by the local ethics committee <strong>of</strong><br />
Hels<strong>in</strong>ki Central Hospital. All subjects gave their written <strong>in</strong>formed consent before the<br />
start <strong>of</strong> the study. Use <strong>of</strong> <strong>tacrolimus</strong> o<strong>in</strong>tment <strong>in</strong> Studies I, II, and V was permitted by<br />
the National Agency for Medic<strong>in</strong>es (Lääkelaitos). Studies I, II, and V were s<strong>in</strong>gle-center<br />
studies conducted <strong>in</strong> the context <strong>of</strong> prospective, multicenter, open-label, noncomparative,<br />
long-term safety studies <strong>in</strong> patients with moderate-to-severe AD us<strong>in</strong>g<br />
<strong>tacrolimus</strong> 0.1% o<strong>in</strong>tment twice daily as an <strong>in</strong>termittent treatment. Study I <strong>in</strong>cluded 6month<br />
and 12-month populations. Study II lasted for 12 to 24 months, and Study V for<br />
48 months. Study III was a cross-section study <strong>of</strong> AD patients and Study IV a<br />
retrospective study <strong>of</strong> patients with <strong>atopic</strong> blepharoconjunctivitis who were us<strong>in</strong>g 0.03%<br />
<strong>tacrolimus</strong> o<strong>in</strong>tment.<br />
Subjects<br />
Male and female patients over 18 years <strong>of</strong> age were eligible for Studies I, II, and IV.<br />
Studies III and V <strong>in</strong>cluded patients over age 13. For more characteristics, see Table 3.<br />
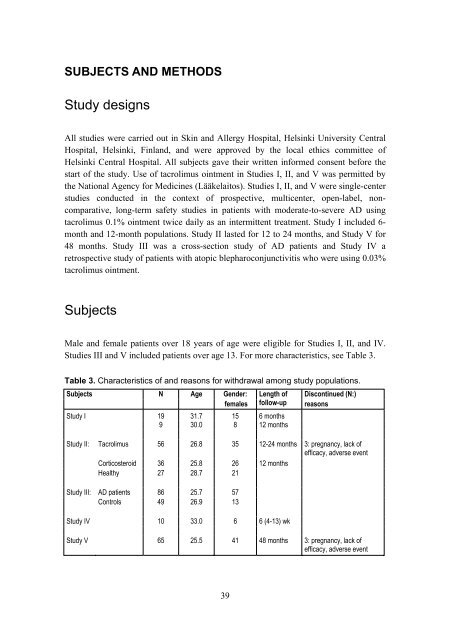
Table 3. Characteristics <strong>of</strong> and reasons for withdrawal among study populations.<br />
Subjects N Age<br />
Gender: Length <strong>of</strong> Discont<strong>in</strong>ued (N:)<br />
females follow-up reasons<br />
Study I 19 31.7 15 6 months<br />
9 30.0 8 12 months<br />
Study II: Tacrolimus 56 26.8 35 12-24 months 3: pregnancy, lack <strong>of</strong><br />
ef<strong>fi</strong>cacy, adverse event<br />
Corticosteroid 36 25.8 26 12 months<br />
Healthy 27 28.7 21<br />
Study III: AD patients 86 25.7 57<br />
Controls 49 26.9 13<br />
Study IV 10 33.0 6 6 (4-13) wk<br />
Study V 65 25.5 41 48 months 3: pregnancy, lack <strong>of</strong><br />
ef<strong>fi</strong>cacy, adverse event<br />
39