Geant4 Simulations for the Radon Electric Dipole Moment Search at
Geant4 Simulations for the Radon Electric Dipole Moment Search at
Geant4 Simulations for the Radon Electric Dipole Moment Search at
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Occupied<br />
Orbitals<br />
Fine<br />
Structure<br />
Hyperfine<br />
Structure<br />
Zeeman<br />
Effect<br />
2<br />
P3/2<br />
5p<br />
F = 3<br />
m<br />
+3<br />
F<br />
2<br />
P1/2<br />
F = 2<br />
_<br />
3<br />
_ 2<br />
+ 2<br />
D 1<br />
D 2<br />
F = 3<br />
+ 3<br />
5s<br />
2<br />
S1/2<br />
F = 2<br />
_ 3<br />
_ 2<br />
+ 2<br />
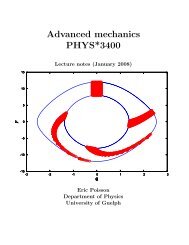
Figure 2.4: Level diagram of 85 Rb (not drawn to scale).<br />
(<strong>the</strong> selection rule <strong>for</strong> this process is ∆m = ±1 or 0 since <strong>the</strong> emitted photon can<br />
have any polariz<strong>at</strong>ion), only <strong>the</strong> m = −1/2 level can absorb <strong>the</strong> incident polarized<br />
photons. The result is th<strong>at</strong> <strong>the</strong> <strong>at</strong>oms become trapped in <strong>the</strong> m = +1/2 ground-st<strong>at</strong>e<br />
level, this process is called “optical pumping” and can produce a very high degree of<br />
polariz<strong>at</strong>ion with almost all <strong>at</strong>oms occupying <strong>the</strong> m = +1/2 magnetic subst<strong>at</strong>e of <strong>the</strong><br />
1S 1/2 ground st<strong>at</strong>e.<br />
N<strong>at</strong>ural rubidium ( 85,87 Rb) vapour will be used in <strong>the</strong> RnEDM experiment to<br />
polarize <strong>the</strong> radon nuclei through spin-exchange collisions. The occupied electronic<br />
orbitals of <strong>the</strong> rubidium <strong>at</strong>om in its ground st<strong>at</strong>e are<br />
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s .<br />
The first 36 electrons are in closed sub-shells which gives zero total angular momentum.<br />
The valence electron in <strong>the</strong> 5s orbital behaves similarly to <strong>the</strong> above simplified<br />
19