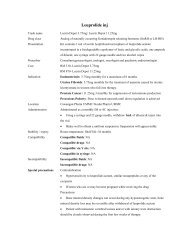
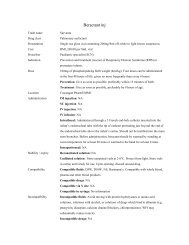
abciximab 10mg inj (IVB IVI)
abciximab 10mg inj (IVB IVI)
abciximab 10mg inj (IVB IVI)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
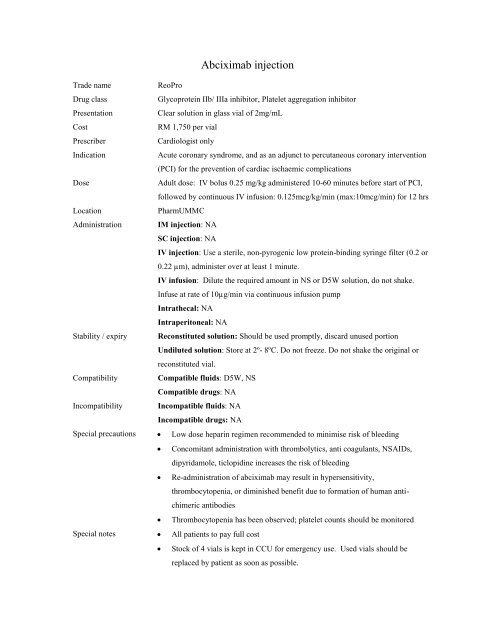
Abciximab <strong>inj</strong>ection<br />
Trade name<br />
ReoPro<br />
Drug class<br />
Glycoprotein IIb/ IIIa inhibitor, Platelet aggregation inhibitor<br />
Presentation<br />
Clear solution in glass vial of 2mg/mL<br />
Cost<br />
RM 1,750 per vial<br />
Prescriber<br />
Cardiologist only<br />
Indication<br />
Acute coronary syndrome, and as an adjunct to percutaneous coronary intervention<br />
(PCI) for the prevention of cardiac ischaemic complications<br />
Dose<br />
Adult dose: IV bolus 0.25 mg/kg administered 10-60 minutes before start of PCI,<br />
followed by continuous IV infusion: 0.125mcg/kg/min (max:10mcg/min) for 12 hrs<br />
Location<br />
PharmUMMC<br />
Administration IM <strong>inj</strong>ection: NA<br />
SC <strong>inj</strong>ection: NA<br />
IV <strong>inj</strong>ection: Use a sterile, non-pyrogenic low protein-binding syringe filter (0.2 or<br />
0.22 µm), administer over at least 1 minute.<br />
IV infusion: Dilute the required amount in NS or D5W solution, do not shake.<br />
Infuse at rate of 10µg/min via continuous infusion pump<br />
Intrathecal: NA<br />
Intraperitoneal: NA<br />
Stability / expiry Reconstituted solution: Should be used promptly, discard unused portion<br />
Undiluted solution: Store at 2º- 8ºC. Do not freeze. Do not shake the original or<br />
reconstituted vial.<br />
Compatibility Compatible fluids: D5W, NS<br />
Compatible drugs: NA<br />
Incompatibility Incompatible fluids: NA<br />
Incompatible drugs: NA<br />
Special precautions Low dose heparin regimen recommended to minimise risk of bleeding<br />
Concomitant administration with thrombolytics, anti coagulants, NSAIDs,<br />
dipyridamole, ticlopidine increases the risk of bleeding<br />
Re-administration of <strong>abciximab</strong> may result in hypersensitivity,<br />
thrombocytopenia, or diminished benefit due to formation of human antichimeric<br />
antibodies<br />
Thrombocytopenia has been observed; platelet counts should be monitored<br />
Special notes All patients to pay full cost<br />
Stock of 4 vials is kept in CCU for emergency use. Used vials should be<br />
replaced by patient as soon as possible.
Patient Information<br />
Prepared/checked by<br />
Date compiled/edition<br />
Reviewed on<br />
If patient dies before replacement is made, the cost of the drug must be<br />
included in the patient’s bill.<br />
GI side effects include nausea and vomiting, hypotension, chest pain, headache<br />
CZS/HKD/MNR/WYY<br />
15 May 2007, 1 st edition; 26 January 2011, 2 nd edition<br />
23 March 2012