immunoglobulin G 2.5g 5g inj (IVI)
immunoglobulin G 2.5g 5g inj (IVI)
immunoglobulin G 2.5g 5g inj (IVI)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
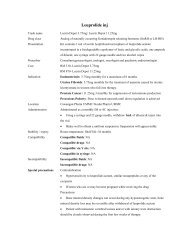
Human Normal Immunoglobulin (<strong>IVI</strong>G)<br />
Trade name<br />
Vigam® Liquid<br />
Drug class<br />
Immunoglobulin<br />
Presentation<br />
Intravenous sterile colourless liquid containing <strong>2.<strong>5g</strong></strong> / vial<br />
Cost<br />
RM320.00 per vial<br />
Approved indications: Govt servants FOC, others – include total cost in hospital<br />
bill if patient is unable to pay.<br />
Prescriber<br />
Consultants & Lecturers<br />
Indication<br />
ITP, Kawasaki disease and paediatric BMT (part of package)<br />
Dose<br />
400mg/kg/day x 5 days or 2g/day x 1 dose<br />
Location<br />
In patient pharmacy<br />
Administration IM <strong>inj</strong>ection: NA<br />
SC <strong>inj</strong>ection: NA<br />
IV <strong>inj</strong>ection: NA<br />
IV infusion: Initial rate, 0.01 – 0.02mL/kg/min for 30min, then gradually increase<br />
to 0.04mL/kg/min up to max rate of 3mL/min<br />
Intrathecal: NA<br />
Intraperitoneal: NA<br />
Stability / expiry Reconstituted solution – Contains no antimicrobial preservatives, use promptly<br />
after reconstitution<br />
Undiluted solution: - Store at 2-8ºC and protect from light. A short period of up<br />
to 3 months at 25ºC is possible within the storage date printed on the label<br />
Compatibility Compatible fluids: NaCl 0.9%, D5%<br />
Compatible drugs: NA<br />
Compatible via Y site: Fluconazole – avoid mixing with other drugs<br />
Compatible in syringe: NA<br />
Incompatibility Incompatible fluids: NA<br />
Incompatible drugs: NA<br />
Special precautions • Adequate hydration is required prior to the infusion of <strong>IVI</strong>G<br />
• Follow recommended infusion rate to minimize adverse reactions<br />
• During the infusion, urine output and serum creatinine levels should be<br />
monitored<br />
• Avoid concomitant use of loop diuretics<br />
• Single use only. Any left over should be discarded<br />
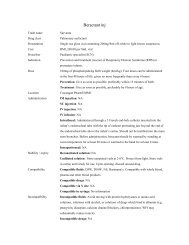
Special notes • Approved indications: All requests for <strong>IVI</strong>G must be accompanied by
Borang Ubat Khas. Completed form does not need to be sent for Director’s<br />
approval. File to be kept in IP. Govt servants FOC, others to pay.<br />
Other indications:<br />
• Govt servants: to pay in full. Those unable to pay cost will need to get the<br />
Borang Ubat Khas form completed and Director’s approval for supply.<br />
• Others: To pay in full. Patients who are unable to pay in full need to fill the<br />
Borang Ubat Khas form and be assessed by the social worker. Completed<br />
forms will be sent for Director’s approval. Patient will be billed for amount<br />
assessed. Copy of approved form must be sent to PTj Kewangan (Hasil)<br />
Patient Information • Adverse reactions include chills, headache, GI side effects, rash and low BP<br />
Prepared/checked by<br />
Date<br />
compiled/edition<br />
• Inform doctor if patient feels unwell during infusion<br />
Tan Wen Chieh / PDS / HKD / PL<br />
23/02/07 / 1 st edition