Insoluble Anodes For The Electroplating Industry - OnBoard ...
Insoluble Anodes For The Electroplating Industry - OnBoard ...
Insoluble Anodes For The Electroplating Industry - OnBoard ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
PCBFABRICATION<br />
<strong>Insoluble</strong> <strong>Anodes</strong><br />
<strong>For</strong> <strong>The</strong> <strong>Electroplating</strong> <strong>Industry</strong><br />
<strong>The</strong> movement towards a new<br />
generation of insoluble anodes<br />
is a very important step for the<br />
future and evolution of the electroplating<br />
industry. “Modified”<br />
insoluble anodes (SIA) have produced<br />
encouraging results in<br />
testing, displaying several advantages<br />
over “standard” insoluble<br />
anodes and suggesting that they<br />
may be suitable for other electroplating<br />
applications such as<br />
CrIII, Zinc and Zinc alloys (such<br />
as Zn-Ni).<br />
by Stéphane Menard,<br />
MPC<br />
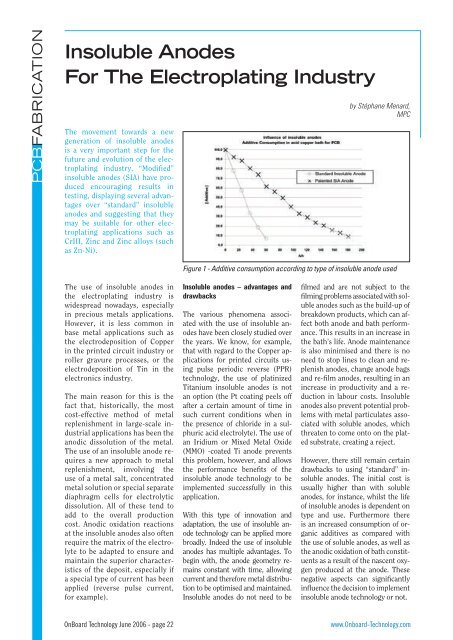
Figure 1 - Additive consumption according to type of insoluble anode used<br />
<strong>The</strong> use of insoluble anodes in<br />
the electroplating industry is<br />
widespread nowadays, especially<br />
in precious metals applications.<br />
However, it is less common in<br />
base metal applications such as<br />
the electrodeposition of Copper<br />
in the printed circuit industry or<br />
roller gravure processes, or the<br />
electrodeposition of Tin in the<br />
electronics industry.<br />
<strong>The</strong> main reason for this is the<br />
fact that, historically, the most<br />
cost-effective method of metal<br />
replenishment in large-scale industrial<br />
applications has been the<br />
anodic dissolution of the metal.<br />
<strong>The</strong> use of an insoluble anode requires<br />
a new approach to metal<br />
replenishment, involving the<br />
use of a metal salt, concentrated<br />
metal solution or special separate<br />
diaphragm cells for electrolytic<br />
dissolution. All of these tend to<br />
add to the overall production<br />
cost. Anodic oxidation reactions<br />
at the insoluble anodes also often<br />
require the matrix of the electrolyte<br />
to be adapted to ensure and<br />
maintain the superior characteristics<br />
of the deposit, especially if<br />
a special type of current has been<br />
applied (reverse pulse current,<br />
for example).<br />
<strong>Insoluble</strong> anodes – advantages and<br />
drawbacks<br />
<strong>The</strong> various phenomena associated<br />
with the use of insoluble anodes<br />
have been closely studied over<br />
the years. We know, for example,<br />
that with regard to the Copper applications<br />
for printed circuits using<br />
pulse periodic reverse (PPR)<br />
technology, the use of platinized<br />
Titanium insoluble anodes is not<br />
an option (the Pt coating peels off<br />
after a certain amount of time in<br />
such current conditions when in<br />
the presence of chloride in a sulphuric<br />
acid electrolyte). <strong>The</strong> use of<br />
an Iridium or Mixed Metal Oxide<br />
(MMO) -coated Ti anode prevents<br />
this problem, however, and allows<br />
the performance benefits of the<br />
insoluble anode technology to be<br />
implemented successfully in this<br />
application.<br />
With this type of innovation and<br />
adaptation, the use of insoluble anode<br />
technology can be applied more<br />
broadly. Indeed the use of insoluble<br />
anodes has multiple advantages. To<br />
begin with, the anode geometry remains<br />
constant with time, allowing<br />
current and therefore metal distribution<br />
to be optimised and maintained.<br />
<strong>Insoluble</strong> anodes do not need to be<br />
filmed and are not subject to the<br />
filming problems associated with soluble<br />
anodes such as the build-up of<br />
breakdown products, which can affect<br />
both anode and bath performance.<br />
This results in an increase in<br />
the bath’s life. Anode maintenance<br />
is also minimised and there is no<br />
need to stop lines to clean and replenish<br />
anodes, change anode bags<br />
and re-film anodes, resulting in an<br />
increase in productivity and a reduction<br />
in labour costs. <strong>Insoluble</strong><br />
anodes also prevent potential problems<br />
with metal particulates associated<br />
with soluble anodes, which<br />
threaten to come onto on the plated<br />
substrate, creating a reject.<br />
However, there still remain certain<br />
drawbacks to using “standard” insoluble<br />
anodes. <strong>The</strong> initial cost is<br />
usually higher than with soluble<br />
anodes, for instance, whilst the life<br />
of insoluble anodes is dependent on<br />
type and use. Furthermore there<br />
is an increased consumption of organic<br />
additives as compared with<br />
the use of soluble anodes, as well as<br />
the anodic oxidation of bath constituents<br />
as a result of the nascent oxygen<br />
produced at the anode. <strong>The</strong>se<br />
negative aspects can significantly<br />
influence the decision to implement<br />
insoluble anode technology or not.<br />
<strong>OnBoard</strong> Technology June 2006 - page 22<br />
www.Onboard-Technology.com
Developing a viable alternative<br />
<strong>The</strong> research and development<br />
work reported in this article was<br />
designed to develop insoluble anodes<br />
that could overcome these<br />
negative aspects and provide users<br />
with a technical and economical<br />
alternative that would enable them<br />
to benefit from the advantages that<br />
this technology offers.<br />
<strong>The</strong> first objective was to focus on<br />
additive consumption at the anodes<br />
and work begun with the investigation<br />
of the acid Copper electrolytes<br />
for PCB applications. <strong>The</strong> following<br />
reaction took place at the insoluble<br />
anode:<br />
2H 2<br />
O => O 2<br />
↑<br />
+ 4e - + 4H +<br />
This reaction, which generates Oxygen<br />
(anodic oxidation), is largely<br />
responsible for the “overconsumption”<br />
of organic additives at the<br />
anode.<br />
A large number of consumption<br />
tests were carried out using different<br />
types of insoluble anode<br />
designs, with additive analysis performed<br />
using the Titraplate CP CVS<br />
instrument. <strong>The</strong> basic approach was<br />
to create a physical barrier between<br />
the anode and the working electrolyte,<br />
essentially achieved by placing<br />
a mesh on top of the anode. <strong>The</strong><br />
mesh acts as a membrane, limiting<br />
the access of the organic substances<br />
to the active anode surface, and<br />
therefore also their destruction.<br />
proved to be much more efficient,<br />
reducing consumption by a factor<br />
of 3. It is therefore clear that an<br />
electrochemical phenomenon is<br />
taking place, meaning that positively<br />
charged compounds can be<br />
repulsed before they reach the active<br />
surface of the anode.<br />
<strong>The</strong> graph in Figure 1 shows the<br />
additive consumption data according<br />
to the type of insoluble anodes<br />
used. <strong>The</strong> very encouraging results<br />
produced with acid Copper led us to<br />
consider these “modified” insoluble<br />
anodes (SIA) for other applications<br />
such as Tin electroplating.<br />
As mentioned previously, with<br />
standard insoluble anodes we observed<br />
a strong oxidation of the Tin<br />
ion at the anode:<br />
Sn 2+ => Sn 4+ + 2 e -<br />
Table 1 compares the formation of<br />
stannic Tin within a MSA matrix of<br />
a pure Tin bath when using standard<br />
insoluble anodes or the modified<br />
insoluble anodes. <strong>The</strong> same results<br />
were confirmed in a sulphuric<br />
acid matrix. <strong>The</strong> principal reason<br />
for this behaviour is the significant<br />
modification of the Oxygen evolution<br />
we observed at the anode using<br />
the SIA insoluble anode. Actually, it<br />
appears that the size and the generation<br />
of the Oxygen bubbles at the<br />
anode are very different from those<br />
observed with the standard insoluble<br />
anode.<br />
Although the phenomenon is difficult<br />
to quantify, it is visibly evident<br />
during plating using the 2 different<br />
types of anodes. Other significant<br />
advantages of using the SIA insoluble<br />
anodes in production have also<br />
been documented. <strong>The</strong>se include<br />
an increase of approximately 10%<br />
in cathode efficiency on the various<br />
installations of the SIA anodes in<br />
operation today, as well as a much<br />
lower oxidative degradation effect<br />
overall and therefore an improvement<br />
in plating bath stability over<br />
time. <strong>The</strong>re are also fewer “bubble<br />
entrapment” problems on applications<br />
requiring the horizontal positioning<br />
of the anode, above or below<br />
the cathode (semiconductor/wafer<br />
or roller-gravure applications), and<br />
an actual increase in anode lifetime<br />
thanks to the SIA concept.<br />
Figure 2 - Example of gassing evolution using insoluble anodes in an acid<br />
Copper electrolyte<br />
PCBFABRICATION<br />
Standard vs. modified insoluble<br />
anodes<br />
Using this approach, jointly patented<br />
by Metakem and MPC (along<br />
with materials used), a specific<br />
mesh/anode configuration was developed<br />
which significantly reduced<br />
additive consumption as compared<br />
with the use of “standard” insoluble<br />
anodes. <strong>The</strong> work also showed that<br />
this barrier is not only physical. An<br />
electrochemical effect was also uncovered<br />
by comparing plastic and<br />
metallic mesh designs. Although<br />
both configurations reduced additive<br />
consumption, the metallic grid<br />
Table 1 – A comparison of the formation of stannic Tin within a MSA<br />
matrix of a pure Tin bath when using standard insoluble anodes and<br />
modified insoluble anodes<br />
www.Onboard-Technology.com<br />
<strong>OnBoard</strong> Technology June 2006 - page 23