P-Based Flame Retardants In Halogen-Free Laminates - OnBoard ...
P-Based Flame Retardants In Halogen-Free Laminates - OnBoard ...
P-Based Flame Retardants In Halogen-Free Laminates - OnBoard ...
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
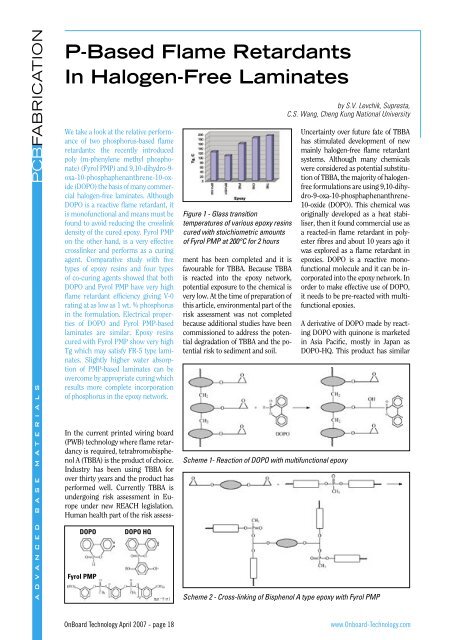
PCBFABRICATIONA D V A N C E D B A S E M A T E R I A L SP-<strong>Based</strong> <strong>Flame</strong> <strong>Retardants</strong><strong>In</strong> <strong>Halogen</strong>-<strong>Free</strong> <strong>Laminates</strong>We take a look at the relative performanceof two phosphorus-based flameretardants: the recently introducedpoly (m-phenylene methyl phosphonate)(Fyrol PMP) and 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide(DOPO) the basis of many commercialhalogen-free laminates. AlthoughDOPO is a reactive flame retardant, itis monofunctional and means must befound to avoid reducing the crosslinkdensity of the cured epoxy. Fyrol PMPon the other hand, is a very effectivecrosslinker and performs as a curingagent. Comparative study with fivetypes of epoxy resins and four typesof co-curing agents showed that bothDOPO and Fyrol PMP have very highflame retardant efficiency giving V-0rating at as low as 1 wt. % phosphorusin the formulation. Electrical propertiesof DOPO and Fyrol PMP-basedlaminates are similar. Epoxy resinscured with Fyrol PMP show very highTg which may satisfy FR-5 type laminates.Slightly higher water absorptionof PMP-based laminates can beovercome by appropriate curing whichresults more complete incorporationof phosphorus in the epoxy network.DOPOFyrol PMPDOPO HQFigure 1 - Glass transitiontemperatures of various epoxy resinscured with stoichiometric amountsof Fyrol PMP at 200°C for 2 hours<strong>In</strong> the current printed wiring board(PWB) technology where flame retardancyis required, tetrabromobisphenolA (TBBA) is the product of choice.<strong>In</strong>dustry has been using TBBA forover thirty years and the product hasperformed well. Currently TBBA isundergoing risk assessment in Europeunder new REACH legislation.Human health part of the risk assessmenthas been completed and it isfavourable for TBBA. Because TBBAis reacted into the epoxy network,potential exposure to the chemical isvery low. At the time of preparation ofthis article, environmental part of therisk assessment was not completedbecause additional studies have beencommissioned to address the potentialdegradation of TBBA and the potentialrisk to sediment and soil.Scheme 1- Reaction of DOPO with multifunctional epoxyby S.V. Levchik, Supresta,C.S. Wang, Cheng Kung National UniversityUncertainty over future fate of TBBAhas stimulated development of newmainly halogen-free flame retardantsystems. Although many chemicalswere considered as potential substitutionof TBBA, the majority of halogenfreeformulations are using 9,10-dihy-dro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO). This chemical wasoriginally developed as a heat stabiliser,then it found commercial use asa reacted-in flame retardant in polyesterfibres and about 10 years ago itwas explored as a flame retardant inepoxies. DOPO is a reactive monofunctionalmolecule and it can be incorporatedinto the epoxy network. <strong>In</strong>order to make effective use of DOPO,it needs to be pre-reacted with multifunctionalepoxies.A derivative of DOPO made by reactingDOPO with quinone is marketedin Asia Pacific, mostly in Japan asDOPO-HQ. This product has similarScheme 2 - Cross-linking of Bisphenol A type epoxy with Fyrol PMP<strong>OnBoard</strong> Technology April 2007 - page 18www.Onboard-Technology.com
Property Fyrol PMP DOPOChemical name Poly(1,3-phenylenemethylphosphonate)9,10-dihydro-9-oxa-10-phosphenanthrene-10-oxideCAS 63747-58-0 35948-25-5Phsophorus 17.5 % 14.3 %Appearance Transparent semi-solid White powderMelting point 50-60°C (handled as a melt at 118°C (handled as a powder)100°C)Functionality Multifunctional MonofunctionalSolubilityApplication withepoxyEpoxy ResinComplementaryHardenerSoluble in MEK and acetone;<strong>In</strong>soluble in toluene or xyleneA curing agent with reactiveequivalent of 90Figure 2 - Glass transitiontemperatures of CNE cured withDICY/Fyrol PMP at 185°C for 2 hoursFigure 3 - Kinetics of pre-reactionof CNE with Fyrol PMP at 3 wt. % PcontentCNE-PMPDICYCNE-PMPPNPCT (30 min) Solder test (dips) 7 >10Moisture abs., % 0.63 0.23PCT (60 min) Solder test 6 >10(times)Moisture abs., % 0.86 0.36Flammability UL-94 V-0 V-0T g (DSC), °C 195 165Soluble in toluene/MEK or xylene/MEK blended solventsMust be pre-reacted with epoxyTable 1 - Physical and chemical properties of Fyrol PMP and DOPOChemicalResistanceMEK Ok OkDMF Ok Ok10% HCl Ok Ok10% NaOH Ok OkTable 2 - Physical properties of laminates based onCNE-PMP (2 wt. % P)functionality as TBBAand can be used as achain extender withdifunctional epoxies.The relatively highcost, but low phosphoruscontent (9.6wt. %) limits wideapplication of DOPO-HQ.The new flame retardant,poly (1,3-phenylenemethylphosphonate),a polymericorganophosphorusproduct is availablefrom Supresta as FyrolPMP. <strong>In</strong> contrastto DOPO or DOPO-HQ, Fyrol PMP isa multifunctional reactive flameretardant (with higher functionalitythan the -OH end groups wouldprovide) and therefore it can be usedeither as a hardener or it can be prereactedwith epoxy.Physical properties of phosphorusflame retardantsSome physical and chemical propertiesof Fyrol PMP and DOPO arelisted in Table 1. Fyrol PMP is a colourlessor slightly amber glassy lowmelting(50-60°C) solid. It is a verythermally stable material and it doesnot show any signs of degradation upto 330ºC. High thermal stability is anindication of the good potential ofFyrol PMP in lead-free soldering formulations.It has very high phosphoruscontent of 17.5% and is solublein MEK and acetone, which are mostcommonly used solvents by the PWBindustry.DOPO is a white hygroscopic powder,which melts at 118°C. DOPO is relativelyhigh in phosphorus (14.3 wt.%), but lower than that of Fyrol PMP.Since DOPO is insoluble in acetoneor MEK, the mixed toluene/MEK orxylene/MEK solvents are used forblending DOPO with epoxy. Pre-reactionof DOPO and epoxy is carried outether in the melt or in a high boilingsolvent. After pre-reaction withepoxy, the resulting resin is solublein acetone or MEK.The reaction of DOPO with epoxyis presented on Scheme 1. BecauseDOPO is a monofunctional reactiveproduct it cannot be used with difunctionalepoxy resins like bisphenolA based epoxy. It must be pre-reactedwith multifunctional epoxies likephenol novolac epoxy (PNE) or cresolnovolac epoxy (CNE). The amount ofDOPO incorporated into epoxy is limitedbecause CNE or PNE resin musthave on average at least two unreactedepoxy groups in order to effectivelycross-link the polymer.Although Fyrol PMP contains someterminal OH functional groups, itsmain reactive functionalities arephosphonate groups, where epoxyinserts in the presence of strong basecatalyst. The curing mechanism isshown in Scheme 2. Every reaction ofthe phosphonate (P-O-C) with epoxyproduces a branch in the polymerchain, which eventually becomes acrosslink. This is usually not the casewith other phosphorus based productsand conventional curing agents.Since each phosphonate group createstwo branching points (potentially twocross-links each) Fyrol PMP shouldbe regarded as a multifunctional. Noaliphatic OH groups are formed inthe course of reaction of Fyrol PMPwith epoxy other than possibly fromthe reaction of the OH end groupswith epoxy. This is very beneficialfor the epoxy resin’s thermal stability,since the thermal decompositionof cured epoxy usually begins withelimination of water from aliphaticOH groups, which further results inbreaking of polymer chains.PCBFABRICATIONA D V A N C E D B A S E M A T E R I A L Swww.Onboard-Technology.com<strong>OnBoard</strong> Technology April 2007 - page 19
PCBFABRICATIONA D V A N C E D B A S E M A T E R I A L SCured epoxy resinsFyrol PMP is a curing agent with reactiveequivalent of about 90. Normallyit cures epoxy resins at 170 – 200°Cin the presence of a base catalyst, like2-methylimidazole (2-MI). Figure 1shows glass transition (Tg) temperaturesof various epoxy resins curedwith a stoichiometric amount of FyrolPMP. As expected, the multifunctionalepoxies (PNE, CNE and TNE(tetrafunctional)) showed higher Tgcompare to difunctional bisphenolA type epoxies (BPA188 (EEW=188)and BPA510 (solid epoxy, EEW=445)).The values of Tg for multifunctionalepoxies can easily accommodate requirementsfor lead-free soldering.Figure 2 shows Tg temperaturesof CNE epoxy resin cured with theblend of DICY and Fyrol PMP. The Tgdecreases upon progressive replacementof DICY with Fyrol PMP. Thisphenomenon is mostly related to thedifferent reactivity of Fyrol PMP andDICY. Because DICY is more reactivethan Fyrol PMP, DICY reacts first andgels epoxy resin relatively fast. Becauseof restrained mobility of polymerchains, Fyrol PMP only partiallyparticipates in the curing process. <strong>In</strong>order to overcome this discrepancy inthe reactivity Fyrol PMP can be prereactedwith epoxy first, prior to curewith DICY.Pre-reaction with CNE epoxy resinSince Fyrol PMP is a multifunctionalreagent, pre-reaction of Fyrol PMP andepoxy should be carried out with caution.Prolonged heating at high temperaturecan result in crosslinking.Figure 3 shows kinetic curves of thepre-reaction of CNE epoxy resin withFyrol PMP at phosphorus content 3wt. %. At 190°C, the reaction proceedsvery fast and the resin approaches thegel point (viscosity greater than 600cp) within only 20 min. The process ismuch more controllable at 150°C.Both Fyrol PMP and DOPO were prereactedwith CNE epoxy resin at 3 wt.% P content and then for some experimentsthe resins were diluted withplain CNE to achieve 1.0 or 2.0 wt.% P content. These epoxy resins wereFigure 4 - Glass transition temperaturesof CNE-DOPO and CNE-PMP curedwith DICY at 185°C for 2 hoursFigure 5 - Water absorption of CNE-DOPO and CNE-PMP cured with DICYFigure 6 - Water absorption of CNE-DOPO and CNE-PMP cured with PNFigure 7 - D kof CNE-DOPO and CNE-PMP cured with DICYFigure 8 - D fof CNE-DOPO and CNE-PMP cured with DICYcured with DICY. Figure 4 shows Tgtemperatures for CNE-DOPO andCNE-PMP. Apparently, Fyrol PMPprovides higher Tg.The water absorption of the sameCNE-DOPO and CNE-PMP formulationsis shown in Figure 5. As expectedthe water absorption increases withincrease of phosphorus content. FyrolPMP shows higher water absorptionthan DOPO. We believe this canbe explained by incomplete reactionof Fyrol PMP with epoxy due to competitionwith more reactive DICY.Tg temperatures of CNE-PMP andCNE-DOPO cured with phenol novolac(PN) range from 160-185°Cdepending on P content and are verysimilar for Fyrol PMP and DOPO. Waterabsorption of CNE-PMP and CNE-DOPO cured with PN is shown in Figure6. Although DOPO shows slightlylower absorption both CNE-DOPOand CNE-PMP have low acceptablevalues. Because the reactivity of PN islower than the reactivity DICY, thereis less competition in curing reactionswith Fyrol PMP, therefore itsincorporation in epoxy is more completeand water absorption is lower.Electrical properties of CNE-PMPand CNE-DOPO cured with DICY areshown in Figues 7 and 8. The dielectricconstant (Dk) and loss factor (Df)were measured in formulations withdifferent P content at 1 and 100 MHz.It is important to note that increase ofphosphorus concentration independentlyof source is beneficial for theepoxy resin because of decrease of Dkand Df. Although both CNE-PMP andCNE-DOPO show comparable valuesof Dk and Df, the values for CNE-PMPtend to be lower.Manufacturing laminatesEight-layer glass cloth laminateswere manufactured using CNE-PMPepoxy resin. P content was adjusted to2 wt. %. Table 2 lists physical propertiesof the laminates cured with DICYand PN respectively. Both laminatesshow strong V-0 UL-94 rating, goodheat resistance in lead-free soldering(288°C) and excellent chemical resistance.The PN cured formulation haslower water absorption and as a resultbetter performance in lead-free soldering;however, the Tg temperatureof the PN-cured formulation is lower.This article is based on a paper originally presentedat the IPC Printed Circuits Expo, APEX,and the Designers Summit 2007<strong>OnBoard</strong> Technology April 2007 - page 20www.Onboard-Technology.com