Stability of Drugs and Dosage Forms Sumie Yoshioka
Stability of Drugs and Dosage Forms Sumie Yoshioka
Stability of Drugs and Dosage Forms Sumie Yoshioka
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
104 Chapter 2 • Chemical <strong>Stability</strong> <strong>of</strong> Drug Substances<br />
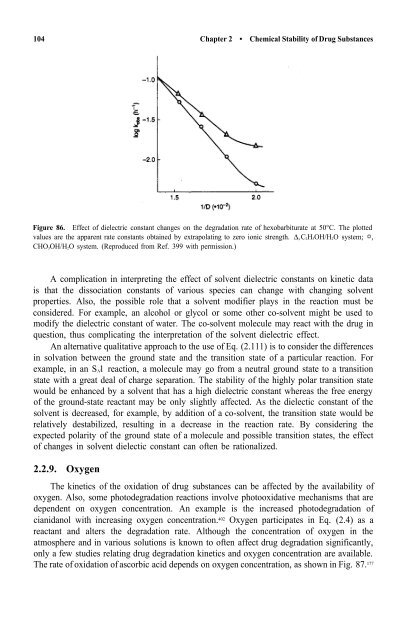
Figure 86. Effect <strong>of</strong> dielectric constant changes on the degradation rate <strong>of</strong> hexobarbiturate at 50°C. The plotted<br />
values are the apparent rate constants obtained by extrapolating to zero ionic strength. ∆, C2H5OH/H2O system; ,<br />
CHO3OH/H2O system. (Reproduced from Ref. 399 with permission.)<br />
A complication in interpreting the effect <strong>of</strong> solvent dielectric constants on kinetic data<br />
is that the dissociation constants <strong>of</strong> various species can change with changing solvent<br />
properties. Also, the possible role that a solvent modifier plays in the reaction must be<br />
considered. For example, an alcohol or glycol or some other co-solvent might be used to<br />
modify the dielectric constant <strong>of</strong> water. The co-solvent molecule may react with the drug in<br />
question, thus complicating the interpretation <strong>of</strong> the solvent dielectric effect.<br />
An alternative qualitative approach to the use <strong>of</strong> Eq. (2.111) is to consider the differences<br />
in solvation between the ground state <strong>and</strong> the transition state <strong>of</strong> a particular reaction. For<br />
example, in an S N 1 reaction, a molecule may go from a neutral ground state to a transition<br />
state with a great deal <strong>of</strong> charge separation. The stability <strong>of</strong> the highly polar transition state<br />
would be enhanced by a solvent that has a high dielectric constant whereas the free energy<br />
<strong>of</strong> the ground-state reactant may be only slightly affected. As the dielectic constant <strong>of</strong> the<br />
solvent is decreased, for example, by addition <strong>of</strong> a co-solvent, the transition state would be<br />
relatively destabilized, resulting in a decrease in the reaction rate. By considering the<br />
expected polarity <strong>of</strong> the ground state <strong>of</strong> a molecule <strong>and</strong> possible transition states, the effect<br />
<strong>of</strong> changes in solvent dielectic constant can <strong>of</strong>ten be rationalized.<br />
2.2.9. Oxygen<br />
The kinetics <strong>of</strong> the oxidation <strong>of</strong> drug substances can be affected by the availability <strong>of</strong><br />
oxygen. Also, some photodegradation reactions involve photooxidative mechanisms that are<br />
dependent on oxygen concentration. An example is the increased photodegradation <strong>of</strong><br />
cianidanol with increasing oxygen concentration. 402 Oxygen participates in Eq. (2.4) as a<br />
reactant <strong>and</strong> alters the degradation rate. Although the concentration <strong>of</strong> oxygen in the<br />
atmosphere <strong>and</strong> in various solutions is known to <strong>of</strong>ten affect drug degradation significantly,<br />
only a few studies relating drug degradation kinetics <strong>and</strong> oxygen concentration are available.<br />
The rate <strong>of</strong> oxidation <strong>of</strong> ascorbic acid depends on oxygen concentration, as shown in Fig. 87. 177