Stability of Drugs and Dosage Forms Sumie Yoshioka
Stability of Drugs and Dosage Forms Sumie Yoshioka
Stability of Drugs and Dosage Forms Sumie Yoshioka
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
5.3. • Degradation Kinetics <strong>of</strong> Peptide <strong>and</strong> Protein Pharmaceuticals 197<br />
dextran, resulting in increased storage stability. 856 In contrast, addition <strong>of</strong> polyvinyl alcohol<br />
with higher mobility lowered the T mc <strong>of</strong> the formulation, resulting in decreased storage<br />
stability. 857<br />
The stability <strong>of</strong> proteins in aqueous solutions is improved by excipients exhibiting<br />
preferential exclusion, such as sugars. 867,868 Porcine growth hormone was stabilized by<br />
HP- β -CD. 869 Denaturation during storage <strong>of</strong> urease <strong>and</strong> interleukin-2 was inhibited by<br />
nonionic surfactants such as poloxamers. 870 Inhibition <strong>of</strong> protein aggregation in solutions<br />
by various sugars <strong>and</strong> surfactants has also been reported for acidic fibroblast growth factor. 871<br />
Stabilizing effects <strong>of</strong> polymeric additives have been reported for low-molecular-weight<br />
urokinase, 872 human I g M monoclonal antibody, 873 <strong>and</strong> human keratinocyte growth factor.<br />
874,875<br />
5.3. Degradation Kinetics <strong>of</strong> Peptide <strong>and</strong> Protein Pharmaceuticals<br />
5.3.1. Quantitative Description <strong>of</strong> Peptide <strong>and</strong> Protein Degradation<br />
Chemical degradation <strong>of</strong> peptide <strong>and</strong> protein pharmaceuticals can also be analyzed<br />
kinetically in the same manner as for small-molecule drugs. Specifically, chemical degradation<br />
<strong>of</strong> small peptides in aqueous solutions generally conforms to simple first-order kinetics.<br />
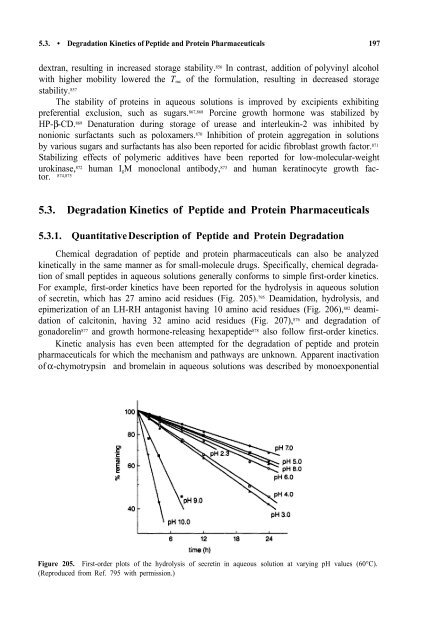
For example, first-order kinetics have been reported for the hydrolysis in aqueous solution<br />
<strong>of</strong> secretin, which has 27 amino acid residues (Fig. 205). 795 Deamidation, hydrolysis, <strong>and</strong><br />
epimerization <strong>of</strong> an LH-RH antagonist having 10 amino acid residues (Fig. 206), 802 deamidation<br />
<strong>of</strong> calcitonin, having 32 amino acid residues (Fig. 207), 876 <strong>and</strong> degradation <strong>of</strong><br />
gonadorelin 877 <strong>and</strong> growth hormone-releasing hexapeptide 878 also follow first-order kinetics.<br />
Kinetic analysis has even been attempted for the degradation <strong>of</strong> peptide <strong>and</strong> protein<br />
pharmaceuticals for which the mechanism <strong>and</strong> pathways are unknown. Apparent inactivation<br />
<strong>of</strong> α -chymotrypsin <strong>and</strong> bromelain in aqueous solutions was described by monoexponential<br />
Figure 205. First-order plots <strong>of</strong> the hydrolysis <strong>of</strong> secretin in aqueous solution at varying pH values (60°C).<br />
(Reproduced from Ref. 795 with permission.)